 | ||
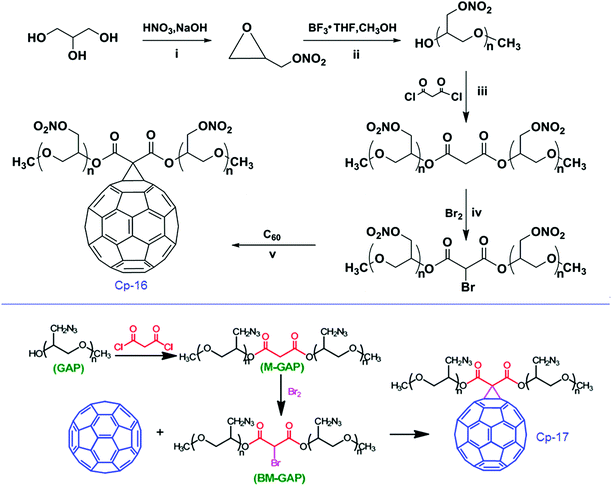
| Scheme 10 Synthesis of C60-PGN and C60-GAP. | ||
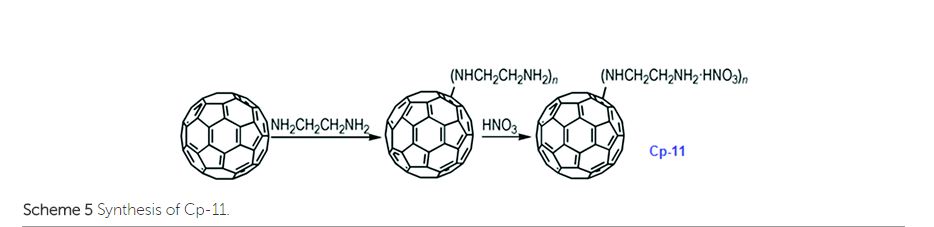
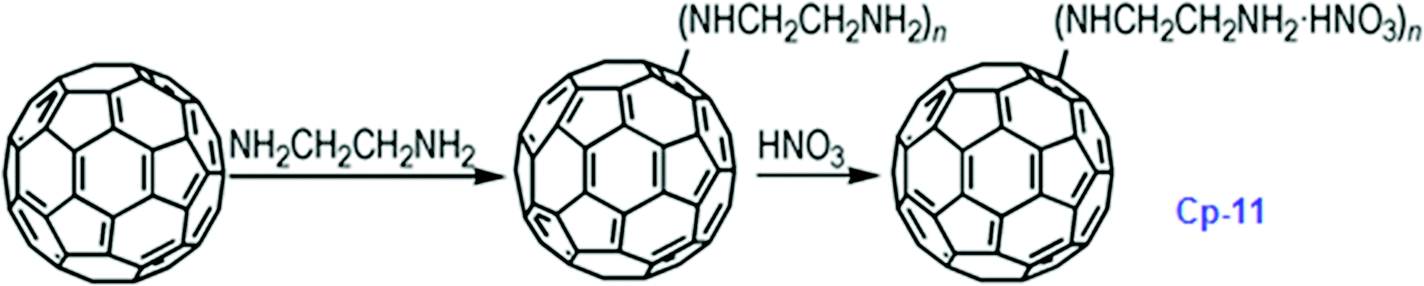
It has been calculated that the enthalpy of formation of Cp-5 is 2782.2 kJ mol−1, while its detonation velocity and pressure are 3282 km s−1 and 4.443 GPa, respectively.112 Upon complete combustion, the total heat of combustion for Cp-5 is 2.17 × 105 kJ mol−1 due to its high carbon content. It seems that this compound may not be useful as the main ingredient of an explosive or a propellant but it might be a good additive for heat-generating energetic compositions. To improve the energetic content of fullerene derivatives, more nitro groups can be introduced on the fullerene skeleton. Such a strategy has been recently attempted, where a high-nitrogen-content derivative, C60(NO2)14 (FP, CP-10), was prepared and characterized,113 using a prolonged treatment of C60 in benzene with very high concentrations of N2O4. However, Cp-10 is not so thermally stable and it deflagrates when heated above 170 °C in nitrogen or air, releasing a considerable amount of heat. This compound may be used as a powerful explosive, depending on its sensitivity, which has not been reported. In addition to the nitro-derivatives of fullerene, fullerene nitrates have also been reported that can be used in propellant compositions as energetic burn rate modifiers. As a typical example, fullerene ethylenediamine nitrate (Cp-11, FEDN) was synthesized by reacting fullerene and ethylenediamine in diluted nitric acid (Scheme ).114
- 112 B. Tan, R. Peng, H. Li, B. O. Jin, S. Chu and X. Long, Theoretical investigation of an energetic fullerene derivative, J. Comput. Chem., 2010, 31, 2233–2237 CAS.
- 113 F. Cataldo, O. Ursini and G. Angelini, Synthesis and explosive decomposition of polynitro[60]fullerene, Carbon, 2013, 62, 413–421 CrossRef CAS.
- 114 B.-L. Chen, B. Jin, R.-F. Peng, F.-Q. Zhao, J.-H. Yi, W.-J. Han, H.-J. Guan and S.-J. Chu, Synthesis and characterization of fullerene-ethylenediamine nitrate, Chin. J. Energet. Mater., 2014, 22, 186–191 CAS.
///////////