Gemfibrozil
CAS: 25812-30-0
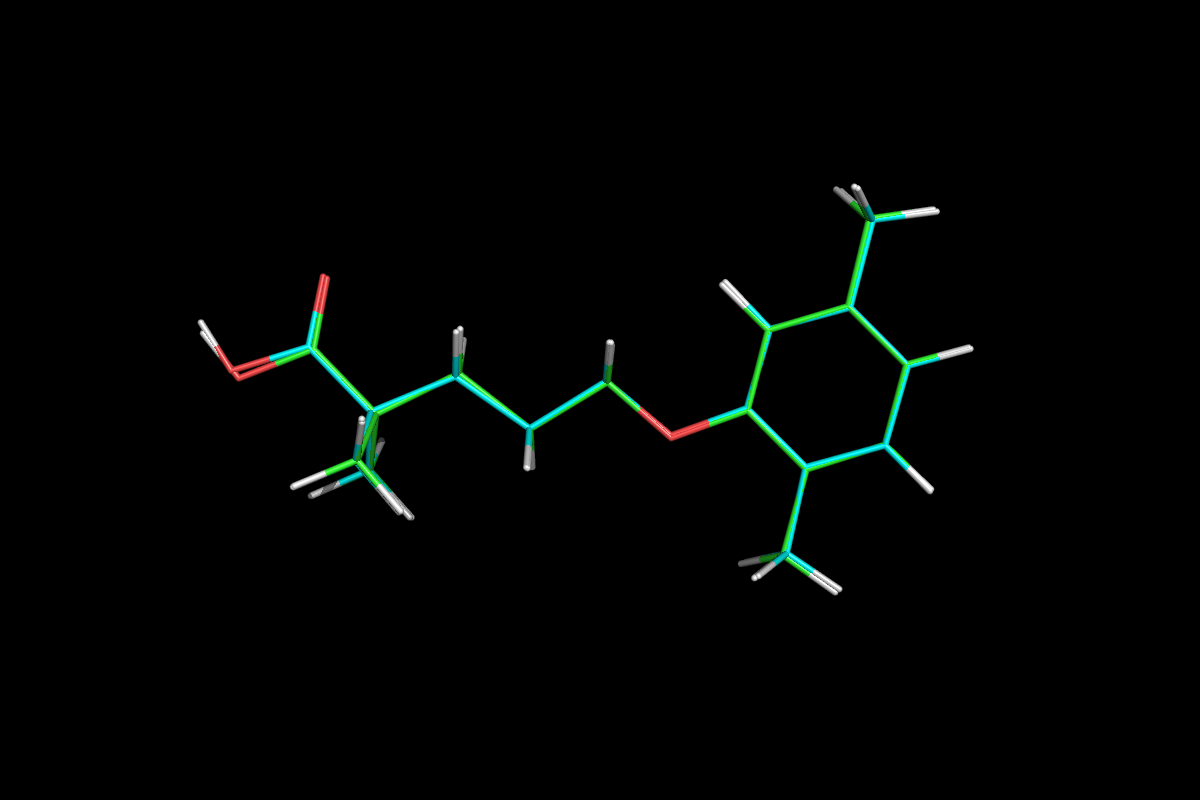
5-(2,5-Dimethylphenoxy)-2,2-dimethylpentanoic acid
2,2-dimethyl-5-(2,5-xylyloxy)valeric acid
Manufacturers' Codes: CI-719
Trademarks: Decrelip (Ferrer); Genlip (Teofarma); Gevilon (Pfizer); Lipozid (Pfizer); Lipur (Pfizer); Lopid (Pfizer)
MF: C15H22O3
MW: 250.33
Percent Composition: C 71.97%, H 8.86%, O 19.17%
Properties: Crystals from hexane, mp 61-63°. bp0.02 158-159°. LD50 in mice, rats (mg/kg): 3162, 4786 orally (Kurtz).
Melting point: mp 61-63°
Boiling point: bp0.02 158-159°
Toxicity data: LD50 in mice, rats (mg/kg): 3162, 4786 orally (Kurtz)
Therap-Cat: Antilipemic.
Gemfibrozil
5-(2,5-Dimethylphenoxy)-2,2-dimethylpentanoic Acid
Gemfibrozil is classified as a fibric acid derivative and is used in the treatment of hyperlipidaemias. It has effects on plasma-lipid concentrations similar to those described under bezafibrate. The major effects of gemfibrozil have been a reduction in plasma-triglyceride concentrations and an increase in high-density lipoprotein (HDL) cholesterol concentrations. A reduction in very-low-density lipoprotein (VLDL)-triglyceride appears to be largely responsible for the fall in plasma triglyceride although reductions in HDL and low-density lipoprotein (LDL)-triglycerides have also been reported.
The effects of gemfibrozil on total cholesterol have been more variable: in general, LDL-cholesterol may be decreased in patients with pre-existing high concentrations and raised in those with low concentrations. The increase in HDL-cholesterol concentrations has resulted in complementary changes to the ratios of HDL-cholesterol to LDL-cholesterol and to total cholesterol. Gemfibrozil has successfully raised HDL-cholesterol concentrations in patients with isolated low levels of HDL-cholesterol but otherwise normal cholesterol concentrations.The Helsinki heart study assessed gemfibrozil for the primary prevention of ischaemic heart disease in middle-aged men with hyperlipidaemia. The usual dose, by mouth, is 1.2 g daily in two divided doses given 30 min before the morning and evening meals. Gemfibrozil is available as tablets for oral administration (Lopid: USP).
IR (KBr, cm–1): 2959.03, 2919.78, 2877.65, 1709.42, 1613.44, 1586.60, 1511.07, 1473.81, 1414.01, 1387.89, 1317.61, 1286.34, 1271.91, 1214.39, 1159.26, 1048.83, 996.57, 803.75;
1H NMR (DMSO, 500 MHz, δ ppm): 1.12 (s, 6H), 1.60 and 1.67 (m, 4H), 2.08 (s, 3H), 2.24 (s, 3H), 3.90 (t, 2H), 6.62 (d, 1H), 6.70 (s, 1H), 6.97 (d, 1H);
13C NMR and DEPT (DMSO, 500 MHz, δ ppm): 15.39 (CH3), 20.94 (CH3), 24.67 (CH2), 24.87 (CH3, CH3), 36.43 (CH2), 40.91 (C), 67.57 (CH2), 112.07 (CH), 120.45 (CH), 122.44 (C), 129.96 (CH), 135.93 (C), 156.43 (C), 178.56 (C);
MS M/Z (ESI): 251.16 [(MH)+].
Solvent:CDCl3Instrument Type:JEOLNucleus:1HFrequency:400 MHzChemical Shift Reference:TMS
1H NMR spectrum of C15H22O3 in CDCL3 at 400 MHz
Gemfibrozil is the
generic name for an oral drug used to lower
lipid levels. It belongs to a group of drugs known as
fibrates. It is most commonly sold as the brand name,
Lopid. Other brand names include
Jezil and
Gen-Fibro.
Gemfibrozil was selected from a series of related compounds synthesized in the laboratories of the American company
Parke Davisin the late 1970s. It came from research for compounds that lower plasma lipid levels in humans and in animals.
[1]
Actions
Therapeutic effects
Nontherapeutic effects and toxicities
Indications
Contraindications and precautions
- Gemfibrozil should not be given to these patients:
- Gemfibrozil should be used with caution in these higher risk categories:
- Biliary tract disease
- Renal dysfunction
- Pregnant women
- Obese patients
Drug interactions
- Anticoagulants: Gemfibrozil potentiates the action of warfarin and indanedione anticoagulants.[citation needed]
- Statin drugs: Concomitant administration of fibrates (including gemfibrozil) with statin drugs increases the risk of muscle cramping, myopathy, andrhabdomyolysis.
- Gemfibrozil inhibits the activation of the liver's Cytochrome P450 system, reducing hepatic metabolism of many drugs, and prolonging their half lives and duration of action.
- Drugs metabolized by the Cytochrome P450 system include:
Environmental data
Gemfibrozil has been detected in
biosolids (the solids remaining after wastewater treatment) at concentrations up to 2650 ng/g wet weight.
[3] This indicates that it survives the wastewater treatment process.
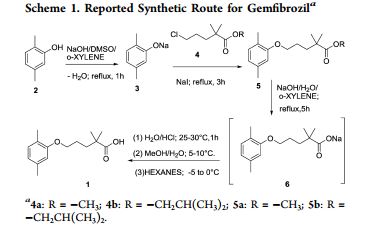
SYNTHESIS
The sodium isobutyrate (I) is metallated with lithium diisopropylamide, and the resulting compound is alkylated with 3- (2,5-dimethylphenoxy) propyl bromide.
PATENT
Paul, L. C. 2,2-Dimethyl-ω-aryloxy alkanoic acids and salts and ester thereof. U.S. 3,674,836, 1972.
CLIP
Production of Gemfibrozil(1)2,5-Dimethylphenol and 1-Bromo-3-chloropropane reaction of 1-(2,5-dimethylphenoxy)-3-chloropropane. The reaction is carried out in toluene, adding new clean off reflux 5h. Just as follows:
(2)N/A can be used to manufacture Gemfibrozil.
PAPER
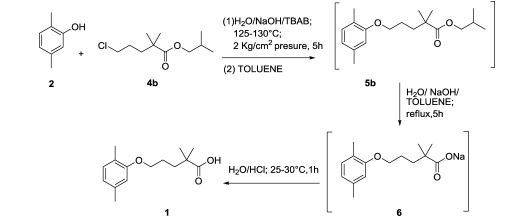
Improved Process for Preparation of Gemfibrozil, an Antihypolipidemic
† Chemical Research and Development, Aurobindo Pharma Ltd., Survey No. 71 and 72, Indrakaran (V), Sangareddy (M), Medak District-502329, Andhra Pradesh, India
‡ Engineering Chemistry Department, AU College of Engineering, Andhra University, Visakhapatnam-530003, Andhra Pradesh, India
Org. Process Res. Dev., 2013, 17 (7), pp 963–966
An improved process for the preparation of gemfibrozil, an antihypolipodimic drug substance, with an overall yield of 80% and ∼99.9% purity (including three chemical reactions) is reported. Formation and control of possible impurities are also described. Finally, gemfibrozil is isolated from water without any additional solvent purification.
Literature References:
Serum lipid regulating agent. Prepn: P. L. Creger, DE 1925423; eidem, US 3674836 (1969, 1972, both to Parke, Davis).
Production: O. P. Goel, US 4126637 (1978 to Warner-Lambert).
Pharmacology: A. H. Kissebach et al.,Atherosclerosis 24, 199 (1976); M. T. Kahonen et al., ibid. 32, 47 (1979).
Series of articles on metabolism, clinical pharmacology, kinetics and toxicology: Proc. R. Soc. Med. 69, Suppl 2, 1-120 (1976).
Toxicity data: S. M. Kurtz et al., ibid. 15.
Clinical trial in hyperlipidemia: J. E. Lewis et al., Pract. Cardiol. 9, 99 (1983).
Clinical reduction of cardiovascular risk in patients with low HDL levels: H. B. Rubins et al., N. Engl. J. Med. 341, 410 (1999).
References
External links
LOPID® (gemfibrozil tablets, USP) is a
lipid regulating agent. It is available as tablets for oral administration. Each tablet contains 600 mg gemfibrozil. Each tablet also contains calcium stearate, NF; candelilla wax,
FCC; microcrystalline cellulose, NF; hydroxypropyl cellulose, NF; hypromellose, USP; methylparaben, NF; Opaspray white; polyethylene glycol, NF; polysorbate 80, NF; propylparaben, NF; colloidal silicon dioxide, NF; pregelatinized starch, NF. The chemical name is 5-(2,5-dimethylphenoxy)2,2-dimethylpentanoic acid, with the following structural formula:
The empirical formula is C15H22O3 and the molecular weight is 250.35; the solubility in water and acid is 0.0019% and in dilute base it is greater than 1%. The melting point is 58° –61°C. Gemfibrozil is a white solid which is stable under ordinary conditions.
/////////Gemfibrozil, Antilipemic, Fibrates, 25812-30-0,
CC1=CC(OCCCC(C)(C)C(O)=O)=C(C)C=C1
Grünersee, Grüner See,
Austria
Grüner See is a lake in Styria, Austria in a village named Tragöß. The lake is surrounded by the Hochschwab Mountains and forests. The name "Green Lake" originated because of its emerald-green water.
Wikipedia
////////