MOXIFLOXACIN
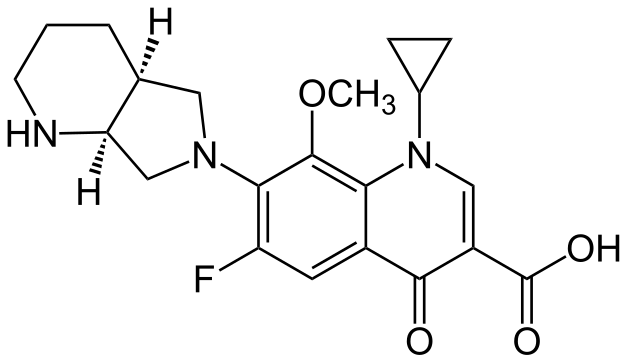
syn.......http://newdrugapprovals.org/2014/02/19/moxifloxacin-bay-12-8039/
1H NMR AND 13C IN DMSO.D6
13C ASSIGN
1H NMR ASSIGN
syn.......http://newdrugapprovals.org/2014/02/19/moxifloxacin-bay-12-8039/
Vietnam Journal of Chemistry
A practical synthesis of fluoroquinolone antibiotic moxifloxacin
206-208 oC;
1H-NMR
(500 MHz, DMSO-d6) δ (ppm): 8.62 (s, 1H, H-2);
7.60 (d, J = 14.5 Hz, 1H, H-5); 4.12-4.10 (m, 1H, H-
13); 3.98-3.94 (m, 1H); 3.88-3.86 (m, 1H); 3.54 (s,
3H, H-12); 3.37-3.23 (m, 3H); 2.88-2.86 (m, 1H);
2.54-2.51(m, 1H); 2.24 (m, 1H); 1.73- 1.59 (m, 3H);
1.41 (m, 1H); 1.21-1.17 (m, 1H); 1.13-1.08 (m, 1H);
1.02-0.96 (m, 1H); 0.89-0.84 (m, 1H).
13C-NMR
(125MHz, DMSO-d6) δ (ppm): 175.8; 165.9; 153.7;
149.9; 140.2; 137.3; 134.5; 116.4; 106.3; 106.1;
61.1; 58.5; 56.0; 52.1; 44.4; 40.6; 36.3; 22.9; 21.5;
9.6; 8.1. ESI-MS m/z: [M-H]-: 400.
COSY
- Figure 13A: X-ray powder diffraction pattern of Moxifloxacin hydrochloride methylene dichloride solvate.
- Figure 13B: Infrared spectrum of Moxifloxacin hydrochloride methylene dichloride solvate.
- Figure 13C: 1H-NMR spectrum (DMSO-d6, TMS) of Moxifloxacin hydrochloride methylene dichloride solvate.
- Figure 14A: X-ray powder diffraction pattern of anhydrous Moxifloxacin hydrochloride form IV.
- Figure 14B: Infrared spectrum of anhydrous Moxifloxacin hydrochloride form IV.
- Figure 15A: X-ray powder diffraction pattern of Moxifloxacin hydrochloride acetic acid solvate.
- Figure 15B: Infrared spectrum of Moxifloxacin hydrochloride acetic acid solvate.
- Figure 15C: 1H-NMR spectrum (DMSO-d6, TMS) of Moxifloxacin hydrochloride acetic acid solvate.
- Figure 16A: X-ray powder diffraction pattern of Moxifloxacin hydrochloride nitromethane solvate.
- Figure 16B: Infrared spectrum of Moxifloxacin hydrochloride nitromethane solvate.
syn.......http://newdrugapprovals.org/2014/02/19/moxifloxacin-bay-12-8039/
- Figure 16C: 1H-NMR spectrum (DMSO-d6, TMS) of Moxifloxacin hydrochloride nitromethane solvate.
- Example 13
- preparation of Moxifloxacine hydrochloride anhydrous form IVExample13.aMoxifloxacine hydrochloride methylene dichloride solvate
- [0100]5 g (12,5 mmol) Moxifloxacin were prepared according to EP 550903 and dissolved in 50 ml methylene dichloride at room temperature. To this solution 0,9 ml (1,2 equiv.) acetic acid and 2 ml (1,2 equiv.) trimethylchlorosilane were added under stirring. Immediately after addition of the chlorosilane the crystallization started. The suspension was stirred for about 30 min and the precipitate filtered off and dried under vacuum at room temperature to yield 6,3g (94,2%) of the 1:1 solvate of Moxifloxacine hydrochloride with methylene dichloride.
1H-NMR (DMSO-d6): 0.8 - 0.95 (m, 1H), 0.95 - 1.3 (m, 3H), 1.6 - 1.9 (m, 4 H), 2.55 - 2.75 (m, 1H), 2.8 - 3.0 (m, 1H), 3.5 - 3.7 (m with Singlet at 3.6 ppm, 4H), 3.7 - 3.8 (m, 1H), 3.8 - 4.0 (m, 2H), 4.0 - 4.2 (m, 2H), 5.77 (s, 2H), 7.65 (d, J = 14 Hz, 1H), 8.66 (s, 1H), 8.95 (s, broad, 1H), 10.2 (s, broad, 1H), 15.1 (s, broad, 1H).
The singlet at 5.77 ppm corresponds to about one mol methylene dichloride per mol of substance (see Figure 13C).
The XRD pattern of the product is shown in Figure 13A and the infrared spectrum obtained is shown in Figure 13B.
The Moxifloxacine hydrochloride solvate with methylene dichloride is not hygroscopic (no water uptake after 1 day at 33% relative humidity.
Example 13.bMoxifloxacine hydrochloride anhydrous form IV
- [0101]2 g Moxifloxacine hydrochloride methylene dichloride solvate were dried in vacuum at 100°C for about 6 hours yielding 1,75 g of the desolvated anhydrous form IV of Moxifloxacine hydrochloride. The XRD pattern of the product is shown in Figure 14A and the infrared spectrum obtained is shown in Figure 14B.
Example 14preparation of Moxifloxacine hydrochloride solvate with acetic acidExample 14.a
- [0102]3,0 g (7,47 mmol) moxifloxacin were prepared according to EP 550903 and dissolved in 30 ml acetic acid at room temperature. To this solution 2,0 ml (2,1 eq) trimethylchlorosilane were added under stirring. 30 minutes after addition of the chlorosilane the crystallization started. The suspension was stirred for about 3,5 hours and the precipitate filtered off washed with acetonitrile and dried under vacuum at room temperature to yield 3,21g (86,3%) of the 1:1 solvate of Moxifloxacine hydrochloride with acetic acid.
1H-NMR (DMSO-d6): 0.8 - 0.95 (m, 1H), 0.95 - 1.3 (m, 3H), 1.6 - 1.9 (m, 4 H), 1.9 (s, 3H), 2.55 - 2.75 (m, 1H), 2.8 - 3.0 (m, 1H), 3.1 - 3.25 (m, 1H), 3.5 - 3.7 (m with Singlet at 3.6 ppm, 4H), 3.7 - 3.8 (m, 1H), 3.8 - 4.0 (m, 2H), 4.0 - 4.2 (m, 2H), 7.63 (d, J = 14 Hz, 1H), 8.65 (s, 1H), 9.1 (s, broad, 1H), 10.4 (s, broad, 1H), 12.7 (s, broad, 1H).
The singlet at 1.9 ppm corresponds to about one mol acetic acid per mol of substance (see Figure 15C).
The XRD pattern of the product is shown in Figure 15A and the infrared spectrum obtained is shown in Figure 15B.
Example 14.b
- [0103]1,0 g (2,49 mmol) Moxifloxacin were prepared according to EP 550903 and dissolved in 5 ml acetic acid and 5 ml acetonitrile at room temperature. To this solution 0,38 ml (1,2 eq) trimethylchlorosilane were added under stirring.
2 hours after addition of the chlorosilane the crystallization didn't start. To this solution 0,38 ml trimethylchlorosilane were once more added under stirring. The suspension was stored for about 17 hours at 4°C and the precipitate filtered off washed with acetonitrile and dried under vacuum at room temperature to yield 0,93g (75,0%) of the 1:1 solvate of Moxifloxacine hydrochloride with acetic acid.
Example 15preparation of Moxifloxacine hydrochloride solvate with nitromethane
- [0104]0,5 g (1,25 mmol) Moxifloxacin were prepared according to EP 550903 and dissolved in 15 ml nitromethane at appr. 60°C. To this solution 0,14 ml (2 eq) acetic acid and (2 eq) ml trimethylchlorosilane were added under stirring. The suspension was stored for about 17 hours and the precipitate was filtered off, washed with acetone and dried under vacuum at room temperature to yield 0,55g (88,2%) of the 1:1 solvate of Moxifloxacine hydrochloride with nitromethane.
1H-NMR (DMSO-d6): 0.8 - 0.95 (m, 1H), 0.95 - 1.3 (m, 3H), 1.6 - 1.9 (m, 4 H), 2.55 - 2.75 (m, 1H), 2.8 - 3.0 (m, 1H), 3.1 - 3.25 (m, 1H), 3.5 - 3.7 (m with Singlet at 3.6 ppm, 4H), 3.7 - 3.8 (m, 1H), 3.8 - 3.95 (m, 2H), 4.0 - 4.2 (m, 2H), 4.44 (s, 3H), 7.63 (d, J = 14 Hz, 1H), 8.65 (s, 1H), 9.0 (s, broad, 1H), 10.3 (s, broad, 1H), 15.1 (s, broad, 1H).
The singlet at 4.44 ppm corresponds to about one mol nitromethane per mol of substance (see Figure 16C).
The XRD pattern of the product is shown in Figure 16A and the infrared spectrum obtained is shown in Figure 16B..............http://www.google.nl/patents/EP2145890B1?cl=en
moxifloxacin synthesis
syn.......http://newdrugapprovals.org/2014/02/19/moxifloxacin-bay-12-8039/Take a tourTake a tour
TONGA
Kingdom of Tonga
Puleʻanga Fakatuʻi ʻo Tonga

Flag Coat of arms Tonga - Wikipedia, the free encyclopedia
Tonga ([ˈtoŋa]; Tongan: Puleʻanga Fakatuʻi ʻo Tonga), officially the Kingdom of Tonga, is a Polynesian sovereign state and archipelago comprising 177 ...en.wikipedia.org/wiki/Tonga
You may not have heard of Tonga, but if you are an adventure buff, then you are definitely in for a treat. Tonga comprises 150 islands, 36 of which are inhabited and lived by people. These islands are very small, but offer very big in terms of tourism and leisure. Also, the guys love to try the customs of Tonga.
Marriage Ceremonies
To maximize the thrill of the experience, make sure you attend at least one marriage ceremony in Tonga. This should not be too hard as Tonga are very helpful and will be more than willing to invite into their ceremonies and festivities. Marriage ceremonies in Tonga are sensational and they love to attend one.
Food Tonga
The way to eat your food Tonga is certainly very different from the way they eat yours. Given that, do not forget to try the local food in Tonga. One is the Lu Pulu - corned beef with a touch of coconut cream and cooked with taro leaves. There are also local beverages in Tonga, including kava, made from pepper plant.
Tonga Customs
The customs of Tonga are very native and unique. Here you will find that people are very friendly and welcoming. Make sure you try to assimilate their culture and customs are very appreciated in Tonga, and certainly do not want to forget this, not to offend a native of Tonga.
Sports
In Tonga, the most popular sport is rugby. Whether or not you are a rugby player, make sure you play this sport in Tonga. The experience will be very different from their country of origin, given the environment of Tonga in the rugby being played.
Beaches of Tonga
Logically, Tonga is home to a lot of beaches. By far, which means the islands of Tonga 150, at least half of the accessible beaches. Here, you can have activities such as sunbathing, playing or even a picnic with his family.
The lives of animals
Although animals are not in abundance in Tonga, are nevertheless given prime importance. Flying foxes and iguanas are everywhere. You can even treat them as exotic foods, however, this practice is discouraged.
Island Hopping
If you are an adventurous person and likes the idea of going from one island to the other, then Tonga is definitely the best place for you. The Tonga islands are very close to each other. The best thing to do is that you can take a boat from one island to another, allowing the excitement and fun of the Greek islands.
Fun water activities
In Tonga, it is fun. And what's more fun than surfing in the Pacific? The tides in Tonga are perfect for this kind of water fun you should try, and you can try water skiing or canoeing water in these places like this will be fun for you and your family.
Diving in Tonga
Another fun water activities you can try in Tonga is scuba diving. Now, why is diving in Tonga much different than elsewhere? Just because Tonga is located at the bottom of the Pacific Ocean, the sea is abundant wildlife in which you may see water species rare and stunning coral formations through their own eyes.
Mountain Activities
Volcanoes and mountains are in abundance in Tonga. This allows you to have a final trekking experience. In Tonga, such activities are managed in a very safe, and can also get to see volcanoes in a very close view.
Hopping Island
Tongan Food
Tongan Beaches
 PALACE
PALACE

















