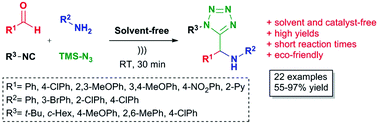
Endogenous water-triggered and ultrasound accelerated synthesis of 1,5-disubstituted tetrazoles via a solvent and catalyst-free Ugi-azide reaction
Green Chem., 2017, Advance Article
DOI: 10.1039/C6GC03324E, Communication
DOI: 10.1039/C6GC03324E, Communication
Shrikant G. Pharande, Alma Rosa Corrales Escobosa, Rocio Gamez-Montano
An ultrasound accelerated, environmentally benign Ugi-azide based method was developed for the synthesis of 1,5-disubstituted tetrazoles under solvent and catalyst-free conditions.
An ultrasound accelerated, environmentally benign Ugi-azide based method was developed for the synthesis of 1,5-disubstituted tetrazoles under solvent and catalyst-free conditions.
Endogenous water-triggered and ultrasound accelerated synthesis of 1,5-disubstituted tetrazoles via a solvent and catalyst-free Ugi-azide reaction
*Corresponding authors
aDepartamento de Química, División de Ciencias Naturales y Exactas, Universidad de Guanajuato, Noria Alta S/N, Col. Noria Alta, Guanajuato, México
E-mail: rociogm@ugto.mx
E-mail: rociogm@ugto.mx
Green Chem., 2017, Advance Article
DOI: 10.1039/C6GC03324E, http://pubs.rsc.org/en/Content/ArticleLanding/2017/GC/C6GC03324E?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract
A novel, sustainable, endogenous water-triggered, environmentally friendly, high substrate scope, efficient, solvent-free and catalyst-free Ugi-azide based method for the synthesis of 1,5-disubstituted tetrazoles is described.


Shrikant Pharande
Research experience
Shrikant Pharande
Research experience
N-((1-(tert-butyl)-1H-tetrazol-5-yl)(4-chlorophenyl)methyl)aniline (4a)
Based on GP, 100 mg 4-Chlorobenzaldehyde (0.71 mmol), 0.065 cm3 aniline (0.71 mmol), 0.080 cm3 ter. Butyl isocyanide (0.71 mmol), and 0.093 cm3 TMS-azide (0.71 mmol) were reacted together to afford 237 mg (97%) as a white solid.
Melting range 144-145oC,
Rf = 0.45 (Hexane-AcOEt = 7/3 V/V),
1H NMR (500 MHz, CDCl3) δ 7.34 – 7.29 (m, 4H), 7.18 – 7.13 (m, 2H), 6.79 – 6.75 (m, 1H), 6.65 (d, J = 7.6 Hz, 2H), 6.11 (d, J = 6.2 Hz, 1H), 4.78 (d, J = 5.6 Hz, 1H), 1.71 (s, 9H);
13C NMR (126 MHz, CDCl3) δ 155.03, 145.54, 136.81, 134.71, 129.62, 129.43, 129.19, 119.64, 114.42, 61.95, 53.93, 30.29;
FT-IR (ATR) νmax/cm-1 3330.5, 3052.5, 2940.9, 1603.6, 1284.1;
HRMS (ESI+): m/z calcd. for C18H20ClN5 + 342.1480, found 342.1474


//////////////
