1613220-15-7 cas
free 1038915-60-4
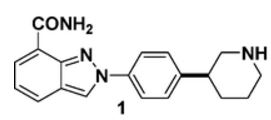
- 2-[4-(3S)-3-Piperidinylphenyl]-2H-indazole-7-carboxamide
- Niraparib
- Jones, Philip; Journal of Medicinal Chemistry 2009, V52(22), P7170-7185
MK-4827
(S)-2-(4-(Piperidin-3-yl)phenyl)-2H-indazole-7-carboxamide Tosylate Monohydrate 1
................... The solid was collected and dried in vacuo at 40 °C to afford 1 as the tosylate monohydrate salt (797 g, 86%, >99 wt %, >99%ee) as a tan-coloured solid.
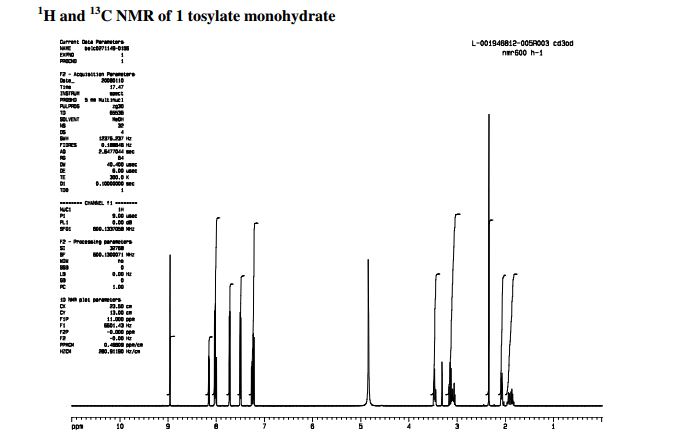
Mp = 144 °C. 1H NMR (600 MHz, CD3OD) δ 8.95 (1H, s), 8.15 (1H, dd, J = 7.1, 1.2 Hz), 8.02 (2H, m), 8.00 (1H, dd, J = 8.3, 1.2 Hz), 7.72 (2H, m), 7.49 (2H, m), 7.25 (1H, dd, J = 8.3, 7.1 Hz), 7.22 (2H, d, J = 8.0 Hz), 3.49–3.43 (2H, m), 3.16–3.04 (3H, m), 2.34 (3H, s), 2.09–2.05 (2H, m), 1.96–1.82 (2H, m).
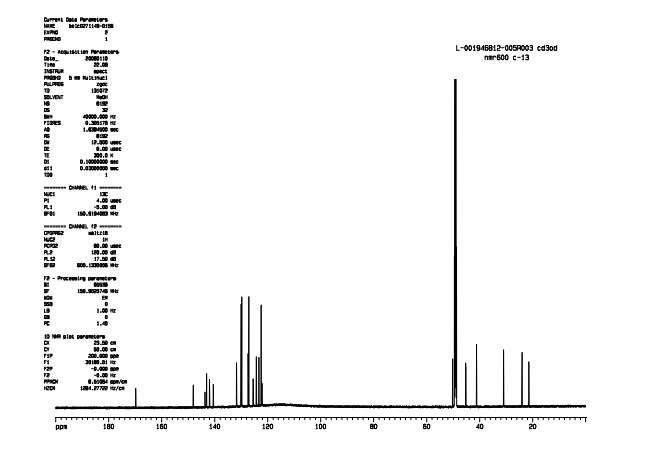
13C NMR (150.9 MHz, CD3OD) δ 169.7, 148.1, 143.7, 143.0, 141.9, 140.5, 131.8, 130.0, 129.8, 127.3, 127.1, 125.4, 124.2, 123.3, 122.4, 50.2, 45.2, 41.1, 30.9, 24.0, 21.4.
Discovery of 2-{4-[(3S)-Piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide (MK-4827): A Novel Oral Poly(ADP-ribose)polymerase (PARP) Inhibitor Efficacious in BRCA-1 and -2 Mutant Tumors
IRBM/Merck Research Labs Rome, Via Pontina km 30,600, 00040 Pomezia, Italy
J. Med. Chem., 2009, 52 (22), pp 7170–7185
DOI: 10.1021/jm901188v, http://pubs.acs.org/doi/abs/10.1021/jm901188v?source=chemport
*To whom correspondence should be addressed. Current address: Department of Medicinal Chemistry, Merck Research Labs Boston, Avenue Louis Pasteur 33, Boston, MA 02115-5727. Phone: +1-617-992-2292. Fax: +1-617-992-2405. E-mail: philip_jones@merck.com.
Abstract

We disclose the development of a novel series of 2-phenyl-2H-indazole-7-carboxamides as poly(ADP-ribose)polymerase (PARP) 1 and 2 inhibitors. This series was optimized to improve enzyme and cellular activity, and the resulting PARP inhibitors display antiproliferation activities against BRCA-1 and BRCA-2 deficient cancer cells, with high selectivity over BRCA proficient cells. Extrahepatic oxidation by CYP450 1A1 and 1A2 was identified as a metabolic concern, and strategies to improve pharmacokinetic properties are reported. These efforts culminated in the identification of 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide 56 (MK-4827), which displays good pharmacokinetic properties and is currently in phase I clinical trials. This compound displays excellent PARP 1 and 2 inhibition with IC50 = 3.8 and 2.1 nM, respectively, and in a whole cell assay, it inhibited PARP activity with EC50 = 4 nM and inhibited proliferation of cancer cells with mutant BRCA-1 and BRCA-2 with CC50 in the 10−100 nM range. Compound 56 was well tolerated in vivo and demonstrated efficacy as a single agent in a xenograft model of BRCA-1 deficient cancer.
//////////1613220-15-7, 1038915-60-4, 2-[4-(3S)-3-Piperidinylphenyl]-2H-indazole-7-carboxamide, Niraparib, mk 4827