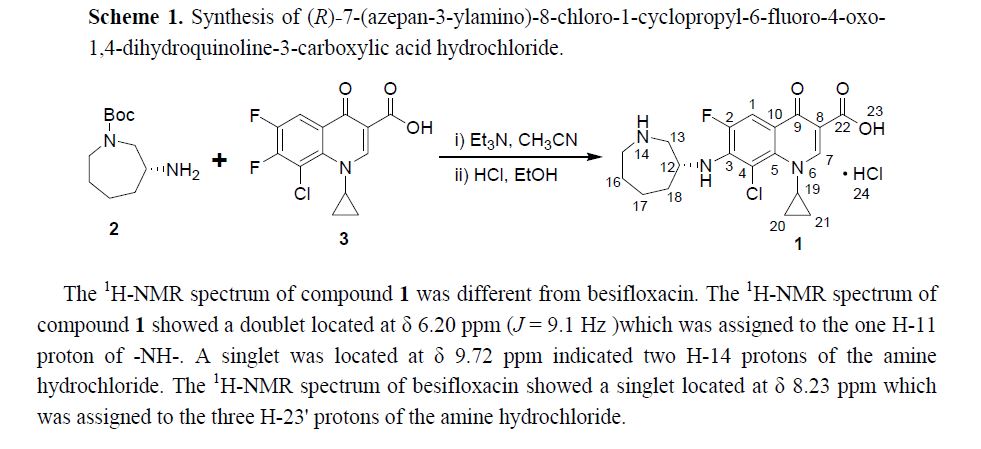
Abstract: In this paper (R)-7-(azepan-3-ylamino)-8-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid hydrochloride 1 was isolated and identified as the N-substituted regioisomer of besifloxacin, which has been synthesized from the reaction of 8-chloro-1-cyclopropyl-6,7-difluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 3 with (R)-tert-butyl 3-aminoazepane-1-carboxylate 2 in acetonitrile as solvent in 37% yield. The chemical structure of compound 1 was established on the basis of 1H-NMR, 13C-NMR, mass spectrometry data and elemental analysis.
Structural Characterization
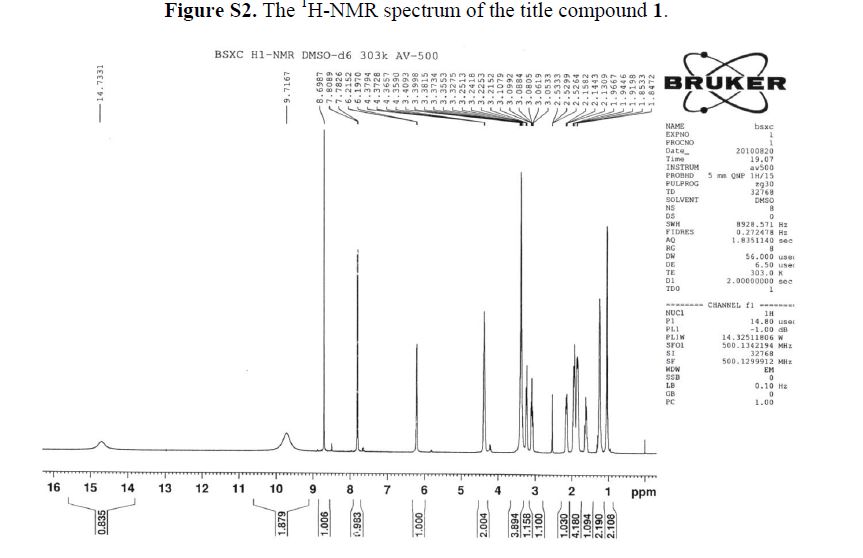
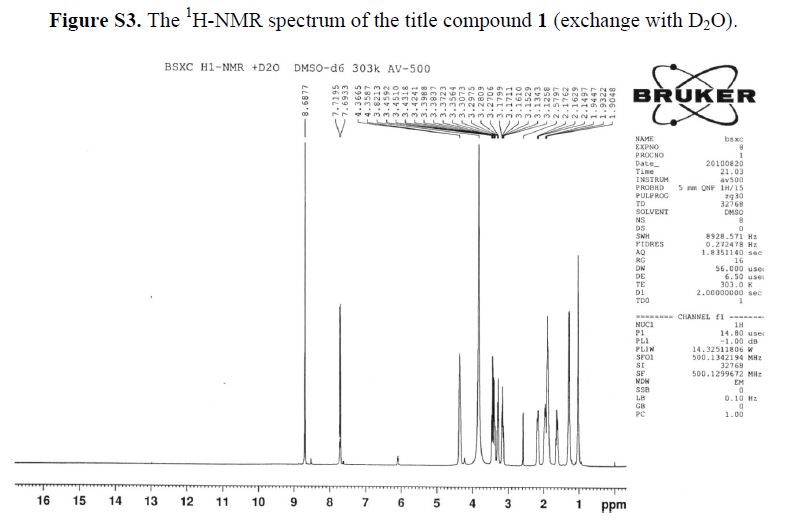
1H-NMR (500 MHz, DMSO-d6): δ ppm: 14.73 (H-23, s, 1H), 9.72 (H-14, s, 2H), 8.69 (H-7, s, 1H),7.79 (H-1, d, J = 13.1 Hz, 1H), 6.20 (H-11, d, J = 9.1 Hz, 1H), 4.37 (H-12 and H-19, m, 2H), 3.38(H-13, m, 2H), 3.23 (H-15, m, 1H), 3.09 (H-15, m, 1H), 2.14 (H-18, m, 1H), 1.94 (H-16 and H-18, m,2H), 1.84 (H-16 and H-17, m, 2H), 1.60 (H-17, m, 1H), 1.23 (H-20 or H-21, m, 2H), 1.03 (H-20 orH-21, m, 2H).
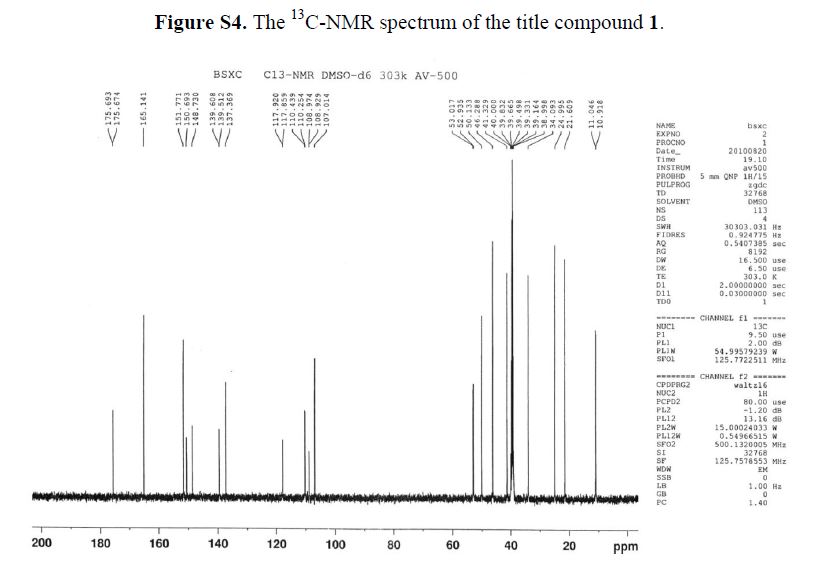
13C-NMR(125 MHz, DMSO-d6): δ ppm: 175.6 (C-9), 165.4 (C-22), 151.7 (C-7), 150.6 (C-2), 148.7(C-3), 139.0 (C-5), 137.3 (C-4), 117.8 (C-10), 110.3 (C-1), 107.0 (C-8), 52.9 (C-12), 50.1 (C-13), 46.2(C-15), 41.3 (C-19), 34.0 (C-18), 24.9 (C-16), 21.6 (C-17), 10.9 (C-20 or C-21).
FAB-MS, m/z = 394.1 (M+).
Elemental analysis: Calculated for C19H21ClFN3O3.HCl: C, 53.03%; H, 5.15%; N, 9.77%; found: C,52.82%; H, 5.39%; N, 9.50%.
1H-NMR (500 MHz, DMSO-d6): δ ppm: 14.73 (H-23, s, 1H), 9.72 (H-14, s, 2H), 8.69 (H-7, s, 1H),7.79 (H-1, d, J = 13.1 Hz, 1H), 6.20 (H-11, d, J = 9.1 Hz, 1H), 4.37 (H-12 and H-19, m, 2H), 3.38(H-13, m, 2H), 3.23 (H-15, m, 1H), 3.09 (H-15, m, 1H), 2.14 (H-18, m, 1H), 1.94 (H-16 and H-18, m,2H), 1.84 (H-16 and H-17, m, 2H), 1.60 (H-17, m, 1H), 1.23 (H-20 or H-21, m, 2H), 1.03 (H-20 orH-21, m, 2H).
13C-NMR(125 MHz, DMSO-d6): δ ppm: 175.6 (C-9), 165.4 (C-22), 151.7 (C-7), 150.6 (C-2), 148.7(C-3), 139.0 (C-5), 137.3 (C-4), 117.8 (C-10), 110.3 (C-1), 107.0 (C-8), 52.9 (C-12), 50.1 (C-13), 46.2(C-15), 41.3 (C-19), 34.0 (C-18), 24.9 (C-16), 21.6 (C-17), 10.9 (C-20 or C-21).
FAB-MS, m/z = 394.1 (M+).
Elemental analysis: Calculated for C19H21ClFN3O3.HCl: C, 53.03%; H, 5.15%; N, 9.77%; found: C,52.82%; H, 5.39%; N, 9.50%.
1H-NMR (500 MHz, DMSO-d6): δ ppm: 14.73 (H-23, s, 1H), 9.72 (H-14, s, 2H), 8.69 (H-7, s, 1H), 7.79 (H-1, d, J = 13.1 Hz, 1H), 6.20 (H-11, d, J = 9.1 Hz, 1H), 4.37 (H-12 and H-19, m, 2H), 3.38 (H-13, m, 2H), 3.23 (H-15, m, 1H), 3.09 (H-15, m, 1H), 2.14 (H-18, m, 1H), 1.94 (H-16 and H-18, m,2H), 1.84 (H-16 and H-17, m, 2H), 1.60 (H-17, m, 1H), 1.23 (H-20 or H-21, m, 2H), 1.03 (H-20 orH-21, m, 2H).
13C-NMR(125 MHz, DMSO-d6): δ ppm: 175.6 (C-9), 165.4 (C-22), 151.7 (C-7), 150.6 (C-2), 148.7(C-3), 139.0 (C-5), 137.3 (C-4), 117.8 (C-10), 110.3 (C-1), 107.0 (C-8), 52.9 (C-12), 50.1 (C-13), 46.2(C-15), 41.3 (C-19), 34.0 (C-18), 24.9 (C-16), 21.6 (C-17), 10.9 (C-20 or C-21).
PAPER
Molbank 2013, 2013(2), M801; doi:10.3390/M801
Short Note
(R)-7-(Azepan-3-ylamino)-8-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid Hydrochloride
Supplementary File 3:Support Information (PDF, 340 KB)
Download PDF [188 KB, 27 May 2013; original version 22 May 2013]
R&D Center, Jiangsu Yabang Pharmaceutical Group, Changzhou 213200, China
In this paper (R)-7-(azepan-3-ylamino)-8-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid hydrochloride 1was isolated and identified as the N-substituted regioisomer of besifloxacin, which has been synthesized from the reaction of 8-chloro-1-cyclopropyl-6,7-difluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 3 with (R)-tert-butyl 3-aminoazepane-1-carboxylate 2in acetonitrile as solvent in 37% yield. The chemical structure of compound 1 was established on the basis of 1H-NMR, 13C-NMR, mass spectrometry data and elemental analysis
 BESIFLOXACIN
BESIFLOXACIN
Zaixin Chen *
R&D Center, Jiangsu Yabang Pharmaceutical Group, Changzhou 213200, China
* Author to whom correspondence should be addressed;
E-Mail: zaixin_chen@163.com.
Zai-Xin Chen
Director of R&D Center at Jiangsu Yabang Pharmaceutical Group Co., Ltd