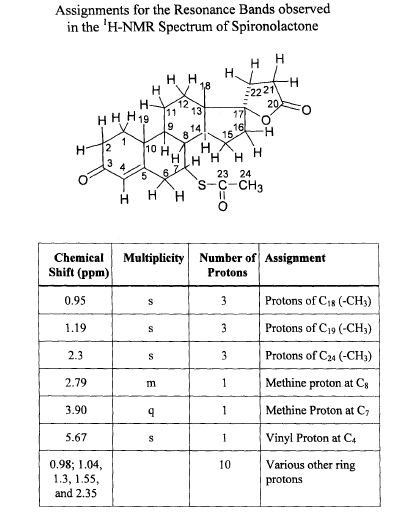
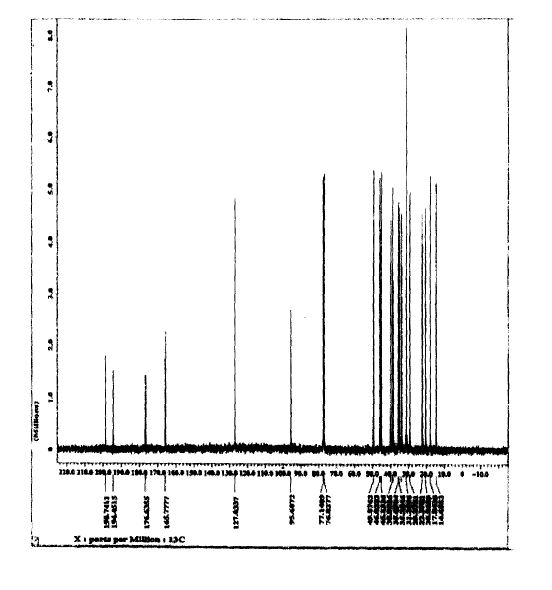
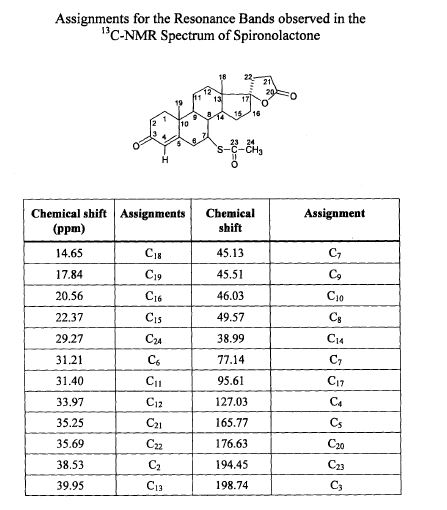
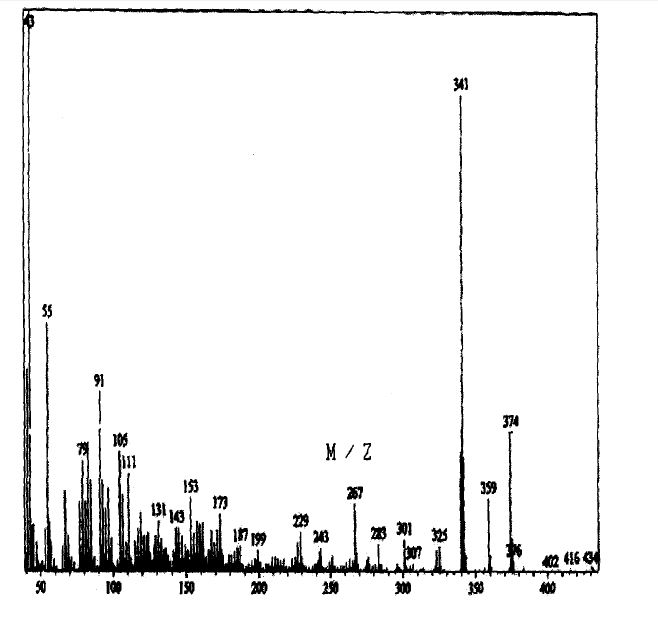
Spironolactone
Spironolactone, Supra-puren, Suracton, спиронолактон, سبيرونولاكتون ,
螺内酯 , Abbolactone, Aldactide, SNL, Spiroctanie, Sprioderm, Verospirone, Opianin
7α-Acetylthio-17α-hydroxy-3-oxopregn-4-ene-21-carboxylic acid γ-lactone
(1'S,2R,2'R,9'R,10'
(7a,17a)-7-(Acetylt
17-Hydroxy-7a-merca
3-(3-Oxo-7a-acetylt
| CAS 52-01-7 |
MF C24H32O4S, MW 416.573 Da
Common side effects include electrolyte abnormalities particularly high blood potassium, nausea, vomiting, headache, a rash, and a decreased desire for sex. In those with liver or kidney problems extra care should be taken.[1]Spironolactone has not been well studied in pregnancy and should not be used to treat high blood pressure of pregnancy.[7] It is a steroid that blocks mineralocorticoid receptors. It also blocks androgen, and blocks progesterone. It belongs to a class of medications known as potassium-sparing diuretics.[1]
Spironolactone was introduced in 1959.[8][9] It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[10] It is available as a generic medication.[1] The wholesale cost in the developing world as of 2014 is between 0.02 and 0.12 USD per day.[11] In the United States it costs about 0.50 USD per day.[1]
Title: Spironolactone
CAS Registry Number: 52-01-7
CAS Name: (7a,17a)-7-(Acetylthio)-17-hydroxy-3-oxopregn-4-ene-21-carboxylic acid g-lactone
Additional Names: 17-hydroxy-7a-mercapto-3-oxo-17a-pregn-4-ene-21-carboxylic acid g-lactone, acetate; 3-(3-oxo-7a-acetylthio-17b-hydroxy-4-androsten-17a-yl)propionic acid g-lactone
Manufacturers' Codes: SC-9420
Trademarks: Aldactone (Pharmacia & Upjohn); Aquareduct (Azupharma); Practon (Pfizer); Osyrol (Aventis); Sincomen (Schering AG); Spirobeta (Betapharm); Spiroctan (Ferlux); Spirolone (APS); Spironone (Dexo); Verospiron (Richter Gedeon); Xenalon (Mepha)
Molecular Formula: C24H32O4S
Molecular Weight: 416.57
Percent Composition: C 69.20%, H 7.74%, O 15.36%, S 7.70%
Literature References: Aldosterone antagonist. Prepn: Cella, Tweit, J. Org. Chem. 24, 1109 (1959); US 3013012 (1961 to Searle); Tweit et al., J. Org. Chem. 27, 3325 (1962). Activity and metabolic studies: Gerhards, Engelhardt, Arzneim.-Forsch. 13, 972 (1963). Crystal and molecular structure: Dideberg, Dupont, Acta Crystallogr. B28, 3014 (1972). Comprehensive description: J. L. Sutter, E. P. K. Lau, Anal. Profiles Drug Subs. 4, 431-451 (1975). Review of carcinogenetic risk: IARC Monographs 24, 259-273 (1980). Review of antiandrogen effects and clinical use in hirsutism: R. R. Tremblay, Clin. Endocrinol. Metab. 15, 363-371 (1986); of clinical efficacy in hypertension: A. N. Brest, Clin. Ther. 8, 568-585 (1986). Review of pharmacology: H. A. Skluth, J. G. Gums,DICP Ann. Pharmacother. 24, 52-59 (1990). Clinical trial in congestive heart failure: B. Pitt et al., N. Engl. J. Med. 341, 709 (1999).
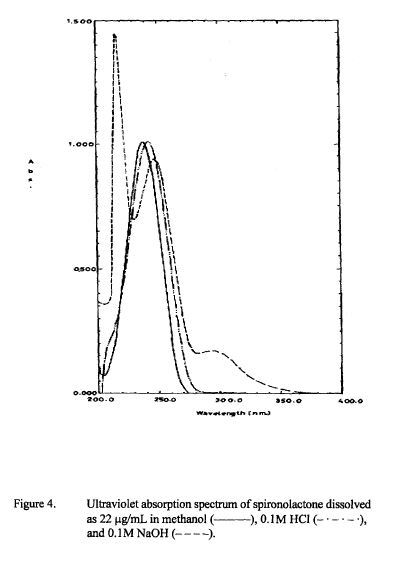
Properties: Crystals from methanol, mp 134-135° (resolidifies and dec 201-202°). [a]D20 -33.5° (chloroform). uv max: 238 nm (e20200). Practically insol in water. Sol in alcohol; freely sol in benzene, chloroform. LD50 in rats, mice, rabbits (mg/kg): 790, 360, 870 i.p. (IARC, 1980).
Melting point: mp 134-135° (resolidifies and dec 201-202°)
Optical Rotation: [a]D20 -33.5° (chloroform)
Absorption maximum: uv max: 238 nm (e 20200)
Toxicity data: LD50 in rats, mice, rabbits (mg/kg): 790, 360, 870 i.p. (IARC, 1980)
Therap-Cat: Diuretic.
Therap-Cat-Vet: Diuretic.
Keywords: Aldosterone Antagonist; Diuretic; Steroids
|
Medical uses
Spironolactone is used primarily to treat heart failure, edematous conditions such as nephrotic syndrome or ascites in people with liver disease, essential hypertension, hypokalemia, secondary hyperaldosteronism (such as occurs with hepatic cirrhosis), and Conn's syndrome (primary hyperaldosteronism). On its own, spironolactone is only a weak diuretic because it primarily targets the distal nephron (collecting tubule), where only small amounts of sodium are reabsorbed, but it can be combined with other diuretics to increase efficacy.
Spironolactone is an antagonist of the androgen receptor (AR) as well as an inhibitor of androgen production. Due to the antiandrogenic effects that result from these actions, it is frequently used off-label to treat a variety of dermatological conditions in which androgens, such as testosterone and dihydrotestosterone (DHT), play a role. Some of these uses include androgenic alopecia in men (either at low doses or as a topical formulation) and women, and hirsutism, acne, and seborrhea in women.[12] Spironolactone is the most commonly used drug in the treatment of hirsutism in the United States.[13] Higher doses of spironolactone are not recommended in males due to the high risk of feminization and other side effects. Similarly, it is also commonly used to treat symptoms of hyperandrogenism in polycystic ovary syndrome.[14]
Spironolactone (SL) is known to be a potent aldosterone antagonist at mineralocorticoid steroid hormone receptors, and it is widely used in humans for the treatment of essential hypertension, congestive heat failure and refractory edema or hyperaldosteronism. However, the prolonged use of SL is associated with undesirable endocrine side effects such as gynecomastia and lose of libido in men and menstrual irregularities in women due to interaction of SL with gonadal steroid hormone biosynthesis and target cell gonadal steroid receptors.
The nature and prevalence of the undesirable side effects limit the usefulness of spironolactone as a therapeutic agent. Gynecomastia or tender breast enlargement has been found to occur in 10% of hypertensive patients using spironolactone for therapy as compared to 1% of men in the placebo group. Recent studies by Pitt, et al. with spironolactone have shown that in patients with congestive heart failure (CHF) taking digoxin and a loop diuretic—spironolactone therapy in conjunction with digitalis and ACE inhibitor—reduces mortality by 30%. See Pitt, B., et al., The Effect of Spironolactone on Morbidity and Mortality in Patients with Severe Heart Failure, Randomized Aldactone Evaluation Study Investigors; N. Engl. J. Med., 1999, 341:709-717. These authors stated that the 30% reduction in the risk of death among patients in the group receiving spironolactone could be attributed to a lower risk of both death from progressive heart failure and sudden death from cardiac arrhythmic causes. In addition, they found that the frequency of hospitalization for worsening heart failure is 35% lower in the spironolacotone treated group than in the placebo group. These authors concluded that patients who received spironolactone had a significant improvement in the symptoms of severe heart failure caused by systolic left ventricular dysfunction. Overall, 8% of the patients in the spironolactone group discontinued treatment because of adverse events. The purpose of the present invention is to make available the individual chiral isomers of spironolactone that would be effective in treating CHF and in reducing hypertension, and at the same time would be devoid of undesirable side effects such as gynecomastia, lose of libido in men, and menstrual irregularities in women.
Spironolactone is the name commonly used for a specific spirolactone that has the full chemical name 17-hydroxy-7-alpha-mercapto-3-oxo-17-alpha-pregn-4-ene-21-carboxylic acid gamma-lactone acetate. The term “spirolactone” denotes that a lactone 10 ring (i.e., a cyclic ester) is attached to another ring structure in a spiro configuration (i.e., the lactone ring shares a single carbon atom with the other ring). Spirolactones that are coupled to steroids are the most important class of spirolactones from a pharmaceutical perspective, so they are widely referred to in the pharmaceutical arts simply as spirolactones. As used herein, “spironolactone” refers to a molecule comprising a lactone structure coupled via a spiro configuration to a steroid structure or steroid derivative.
Spironolactone, its activities, and modes of synthesis and purification are described in a number of U.S. patents, notably U.S. Pat. Nos. 3,013,012, 4,529,811 and 4,603,128.
Intracellular receptors (IRs) form a class of structurally-related genetic regulators that act as ligand-dependent transcription factors. See Evans, R. M., “The Steroid and Thyroid Hormone Receptor Superfamily”, Science, May 13, 1988; 240(4854):889-95. Steroid receptors are a recognized subset of the IRs, including the progesterone receptor (PR), androgen receptor (AR), estrogen receptor (ER), which can be referred to collectively as the gonadal steroid receptors, glucocorticoid receptor (GR), and mineralocorticoid receptor (MR). Regulation of a gene by such factors requires both the IR itself and a corresponding ligand that has the ability to selectively bind to the IR in a way that affects gene transcription.
Ligands for the IRs can include low molecular weight native molecules, such as the hormones aldosterone, progesterone, estrogen and testosterone, as well as synthetic derivative compounds such as medroxyprogesterone acetate, diethylstilbesterol and 19-nortestosterone. These ligands, when present the fluid surrounding a cell, pass through the outer cell membrane by passive diffusion and bind to specific IR proteins to create a ligand/receptor complex. This complex then translocates to the cell's nucleus, where it binds to a specific gene or genes present in the cell's DNA. Once bound to DNA, the complex modulates the production of the protein encoded by that gene. In this regard, a compound that binds to an IR and mimics the effect of the native ligand is referred to as an “agonist”, while a compound that binds to an IR and inhibits the effect of the native ligand is called an “antagonist”.
The therapeutic mechanism of action of spironolactone involves binding to intracellular mineralocorticoid receptors (MRs) in kidney epithelial cells, thereby inhibiting the binding of aldosterone. Spironolactone has been found to counteract the sodium reabsorption and potassium excretion effects of aldosterone and other mineralocorticoids. Spironolactone has also been shown to interfere with testosterone biosynthesis, has anti-androgen action and inhibits adrenal aldosterone biosynthesis. Large doses of spironolactone in children appear to decrease the testosterone production rate.
Spironolactone is found to exhibit intra-individual variability of pharmacokinetic parameters and it presumably belongs to the group of drugs with high inter-subject variability. Spironolactone has poor water solubility and dissolution rate.
In order to prolong the half-life and decrease the side effects associated with spironolactone, syntheses of spironolactone derivatives have been developed (e.g. synthesis of mexrenone, prorenone, spirorenone). Slight modifications of the spironolactone steroid skeleton, e.g. such as formation of 11β-allenic and epoxy compounds, have been shown to effect important variations in the affinity and specificity for the mineralocorticoid receptor. These results suggest that it is possible to develop spironolactone analogues that do not interact with the androgen receptor or cytochrome P-450 and are therefore free of spironolactone undesirable side-effects.
METABOLISM

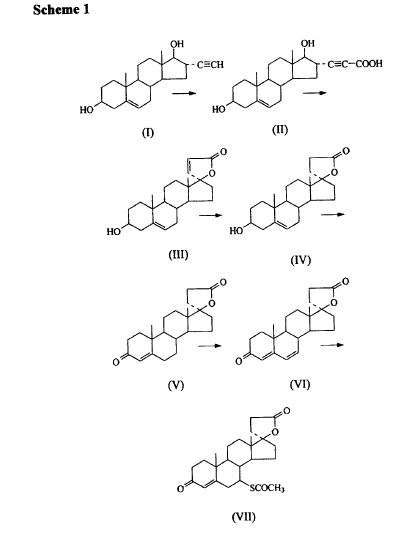
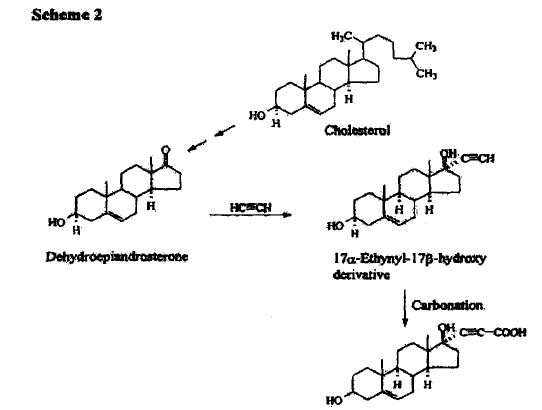
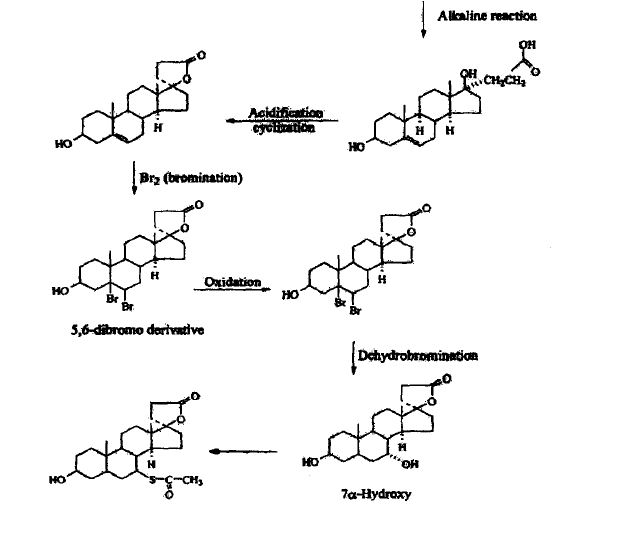
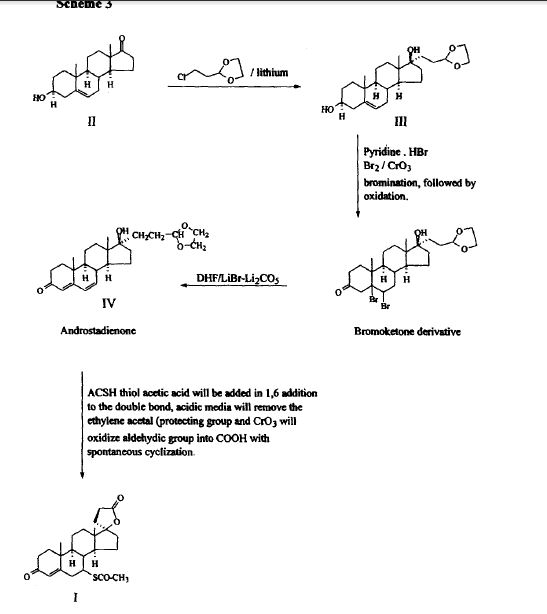
SYNTHESIS
METHOD 1 REF 150
REF 130, 150
METHOD 2 REF 140
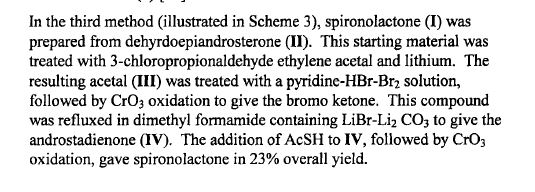
METHOD 3 REF 150
Synthesis
Cella, John A.; Tweit, Robert C. (1959). Journal of Organic Chemistry 24: 1109. doi:.
(See also part 1 and part 3)

SPECTROSCOPY UV
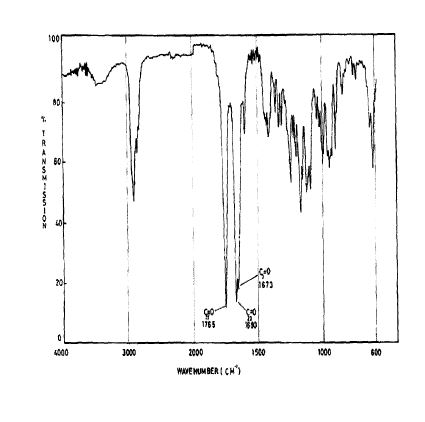
SPECTROSCOPY IR
KBR
The principal absorption peaks of the spectrum shown in Figure 5 were noted at 1765,
1693, 1673, 1240, 1178, 1135, 1123 and 1193 cm -1.
1693, 1673, 1240, 1178, 1135, 1123 and 1193 cm -1.
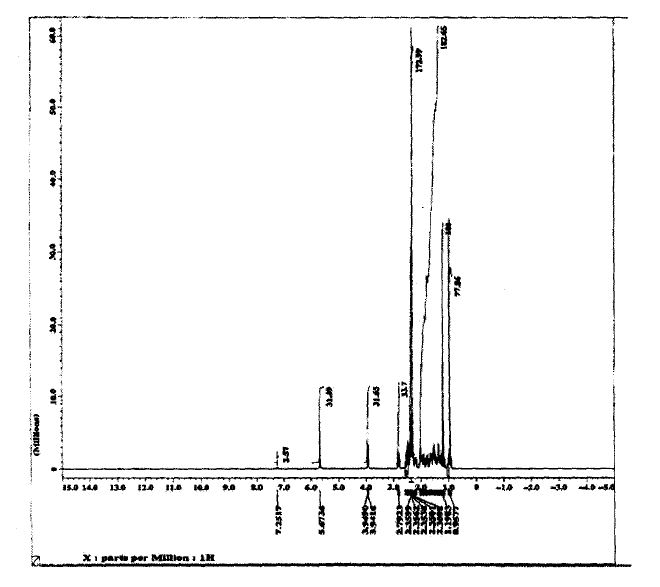
SPECTROSCOPY 1H NMR
SPECTROSCOPY 13C NMR
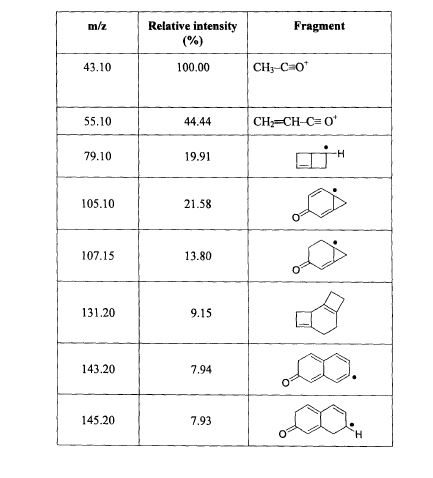
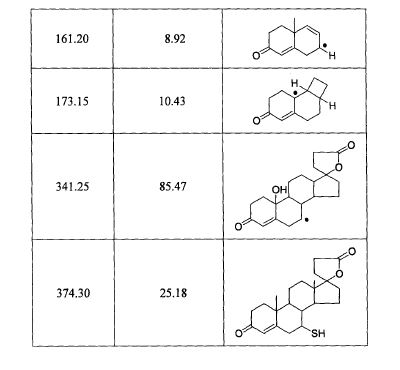
SPECTROSCOPY MASS SPECTRUM
130 J.A. Cola, E.A. Brown, and R.R. Burtner, 3. Org. Chem., 24, 1109(1959).
140 Remington's: The Science and Practice of Pharmacy, 19 t~ edn.Volume II, K.G. Alfonso, ed.; Mack Publishing Co., Pennsylvania (1995) p.1048.
150. G. Anner and H. Wehrli (Ciba-Geigy, A.-G.), German Often 2,625,723 (cl.C07J21/00), Dec,1976; Swiss Appl. 75/7, 696, 13Jun. 1975; pp. 37.
150. G. Anner and H. Wehrli (Ciba-Geigy, A.-G.), German Often 2,625,723 (cl.C07J21/00), Dec,1976; Swiss Appl. 75/7, 696, 13Jun. 1975; pp. 37.
ANALYTICAL
High-Performance Liquid Chromatographic Conditions Column LiChrosorb RP-8, 5 μm. 150 × 4.6 mm I.D. Eluent Acetonitrile-0.05 M phosphate buffer, pH 4 (45:55) Flow-rate 1 ml/min Temperature 25° C. Detector UV detector, wavelength 286 nm or 271 nm Recorder Chart speed 0.5 cm/min Sample loop 10 μl - The concentration of canrenone is determined in plasma and urine samples by high-performance liquid chromatography (HPLC) with UV-detection. An aliquot of 300 ng of spironolactone derivative is added to the samples as internal standard, which are then extracted twice with 1 ml n-hexane-toluene (1:1, v/v). The organic phase is taken to dryness and re-dissolved in 250 μl HPLC eluent (methanol-water, 60:40, v/v). (25×4.6 mm; 5 μm). Detection is performed with the UV detector set at λ=285 nm.
Flurometric Method
- Five ml of water is a reagent blank and 5 ml of working standards containing 0.05 μg and 0.20 μg of SC-9376 are carried through the entire procedure. Lower sales are read vs. the 0.05 μg standard at full scale, and higher samples vs. the 0.20 μg standard. Fluorescence readings are proportional to the concentrations of the standards in this range.
- Pipette 0.2 ml of heparinized plasma into a 50-ml polyethylene-stoppered centrifuge tube, dilute to 5 ml with water and add 15 ml of methylene chloride (Du Pont refrigeration grade, redistilled). Shake for 30 seconds, centrifuge and discard the aqueous supernatant. Add 1 ml 0.1 N NaOH, shake 15 seconds, centrifuge and discard the supernatant. Transfer a 10-ml aliquot of the methylene chloride phase to another tube containing 2 ml of 65% aqueous sulfuric acid, shake 30 seconds, centrifuge and remove organic phase by aspiration. The material is allowed to stand at room temperature for about 1 hour and then about 1 ml of the sulfuric acid phase in transferred to a quartz cuvette. Fluorescence intensity is determined in an Aminco-Bowman spectrophotofluorometer (activation maximum, 465 nm).
- Gas Liquid Chromatography
- The GLC estimation is carried out on a Fractovap Model 251 series 2150 (Carlo Erba) instrument equipped with a Nickel-63 electron capture detector. A 6-foot, 0.4 mm internal diameter, U-shaped glass column, packed with OV-17 2% or XE-60 1% on gas chrom A, 100-120 mesh (Applied Science Lab) is conditioned for 3 days before use. Argon with 10% methane which passed through a molecular sieve before entering the column is used as the carrier gas. The conditions of analysis are: column 255° C., detector 275° C., carrier gas flow 30 ml/min. Samples are injected on the column with a 10 μl Hamilton syringe. The injector in not heated.
PATENT
EXAMPLE 1Chiral Separation
The separation of 7 beta isomer of SL is schematically described below.
- Chromatographic Method for Isolation of SL IsomersThe basic method is described in Chan, Ky, et al., J. Chromatog, Nov. 15, 1991:571 (1-2) 291-297. The separation is performed using spectra-physics HPLC instrument and UV variable wavelength detector set at 254 nm. For chiral separation, the chromatographic column is either a pre-packed 25 mm×4.6 mm ID Cyclobond 1 (5 μm particle size), or a pre-packed 150 mm×4 mm ID Resolvosil BSA-7 column (5 μm) operated using the conditions described herein.Analysis of the isomers present in the peaks in the chromatograms and their chiral extract purity analysis can be determined in each case by high resolution NMR spectroscopy using a chiral shift reagent. Based on this information and the determination of molecular weight by mass spectrometry and/or optical activity, structural configuration is assigned to each isomer. Eluted samples of isomers may be re-chromatographed in order to obtain adequate quantities of isomers having desired optical purity for study. For future use, reference standards that are optically pure will be compared for confirmation of purity and identity to the isolated isomers that are obtained after their chromatographic separation.
EXAMPLE 2Chemical Synthesis of Optical Isomers
- As an example, the desire spironolactone 7-beta-isomer is synthesized following the scheme that is described below:
- Diene (i) is prepared from commercially available starting materials using methods well known in the art of chemical synthesis.Diene (i) is treated with acetic acid and the mixture is heated to reflux to yield 7-alpha-acetate ester (ii). The 7-alpha-ester (ii) is further subjected to nucleophilic substitution, followed by hydrolysis to obtain the 7-beta-isomer (iii). The 7-beta-isomer (iii) is then esterified with an acyl halide in the presence of a base to generate the desired spironolactone 7-beta-isomer (iv).
EXAMPLE 3Preparation of Radiolabeled Probe Compounds of the Invention
- Using known methods, the compounds of the invention may be prepared as radiolabeled probes by carrying out their synthesis using precursors comprising at least one atom that is a radioisotope. The radioisotope is preferably selected from at least one of carbon (preferably
14
- C), hydrogen (preferably
3
- H), sulfur (preferably
35
- S), or iodine (preferably I). Such radiolabeled probes are conveniently synthesized by a radioisotope supplier specializing in customer synthesis of radiolabeled probe compounds. Such suppliers include Amersham Corporation, Arlington Heights, Ill.; Cambridge Isotope Laboratories, Inc., Andover, Mass.; SRI International, Menlo Park, Calif.; Wizard Laboratories, West Sacramento, Calif.; ChemSyn Laboratories, Lexena, Kans.; American Radiolabeled Chemicals, Inc., St. Louis, Mo.; and Moravek Biochemicals Inc., Brea, Calif.
- Tritium labeled probe compounds are also conveniently prepared catalytically via platinum-catalyzed exchange in tritiated acetic acid, acid-catalyzed exchange in tritiated trifluoroacetic acid, or heterogeneous-catalyzed exchange with tritium gas. Tritium labeled probe compounds can also be prepared, when appropriate, by sodium borotritide reduction. Such preparations are also conveniently carried out as a custom radiolabeling by any of the suppliers listed in the preceding paragraph using the compound of the invention as substrate.
- EXAMPLE 4Isolation and Purification Procedure
- The optical isomers of spironolactones may be isolated from fluid sample such as urine or blood as follows:
- Extraction from Urine
- The urine sample is extracted with dichloromethane and the extract washed with NaOH (0.1 N) and then with water to neutrality. The residue obtained after evaporation of the dichloromethane extract is purified on TLC in three different systems: benzene-acetone-water, (150:100:0.4); chloroform-ethanol, (90:10); ethyl acetate-cyclohexane-ethanol, (45:25:10), using aldosterone as reference standard.
- The extract is then purified by high performance liquid chromatography (HPLC) on a Waters 6000 A, 480 U.V. detector instrument with radial pressure. The extract is first run through a C
18
- 10μ column using methanol-water (70:30) as the eluent, followed by a silica 5μ column using dichloromethane-methanol (95:5). In both cases, the rate of the eluent is 1.5 ml/min. A small part of the extract is subjected to heptafluorobutyrylation for GLC investigation.
References
- "Spironolactone". The American Society of Health-System Pharmacists. Retrieved Oct 24, 2015.
- "Spironolactone: MedlinePlus Drug Information". Retrieved 2016-01-20.
- "Spironolactone". Merriam-Webster Dictionary.
- "Spironolactone". Dictionary.com Unabridged. Random House.
- Harry G. Brittain (26 November 2002). Analytical Profiles of Drug Substances and Excipients. Academic Press. p. 309. ISBN 978-0-12-260829-2. Retrieved 27 May 2012.
- Maizes, Victoria (2015). Integrative Women's Health (2 ed.). p. 746.ISBN 9780190214807.
- "Spironolactone Pregnancy and Breastfeeding Warnings". Retrieved 29 November2015.
- Camille Georges Wermuth (24 July 2008). The Practice of Medicinal Chemistry. Academic Press. p. 34. ISBN 978-0-12-374194-3. Retrieved 27 May 2012.
- Marshall Sittig (1988). Pharmaceutical Manufacturing Encyclopedia. William Andrew. p. 1385. ISBN 978-0-8155-1144-1. Retrieved 27 May 2012.
- "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- "Spironolactone". International Drug Price Indicator Guide. Retrieved 29 November2015.
- Hughes BR, Cunliffe WJ (May 1988). "Tolerance of spironolactone". The British Journal of Dermatology 118 (5): 687–91. doi:10.1111/j.1365-2133.1988.tb02571.x.PMID 2969259.
- Victor R. Preedy (1 January 2012). Handbook of Hair in Health and Disease. Springer Science & Business Media. pp. 132–. ISBN 978-90-8686-728-8.
- Loy R, Seibel MM (December 1988). "Evaluation and therapy of polycystic ovarian syndrome". Endocrinology and Metabolism Clinics of North America 17 (4): 785–813.PMID 3143568.
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
| 7α-Acetylthio-17α-hydroxy-3-oxopregn-4-ene-21-carboxylic acid γ-lactone | |
| Clinical data | |
| Pronunciation | /spɪˌroʊnəˈlæktoʊn, spaɪ-, spə-, -ˈrɒ-, -noʊ-/or /ˌspaɪrənoʊˈlæktoʊn/[2][3][4] |
| Trade names | Aldactone |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682627 |
| Pregnancy category | |
| Routes of administration | Oral[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 90%+[5] |
| Metabolism | Hepatic CYP450 |
| Biological half-life | 1.3-2 hours |
| Excretion | Urine, bile |
| Identifiers | |
| CAS Number | 52-01-7 |
| ATC code | C03DA01 (WHO) |
| PubChem | CID 5833 |
| IUPHAR/BPS | 2875 |
| DrugBank | DB00421 |
| ChemSpider | 5628 |
| UNII | 27O7W4T232 |
| KEGG | D00443 |
| ChEBI | CHEBI:9241 |
| ChEMBL | CHEMBL1393 |
| Chemical data | |
| Formula | C24H32O4S |
| Molar mass | 416.574 g/mol |
///////Spironolactone, Supra-puren, Suracton, спиронолактон, سبيرونولاكتون ,
螺内酯 , Abbolactone, Aldactide, SNL, Spiroctanie, Sprioderm, Verospirone, Opianin
O=C5O[C@@]4([C@@]3([C@H]([C@@H]2[C@H](SC(=O)C)C/C1=C/C(=O)CC[C@]1(C)[C@H]2CC3)CC4)C)CC5