
Tenofovir Disoproxil Fumarate
SYN...........
https://newdrugapprovals.org/2016/03/15/an-improved-process-for-the-preparation-of-tenofovir-disoproxil-fumarate/
NMR.........http://file.selleckchem.com/downloads/nmr/s140001-tenofovir-disoproxil-fumarate-hnmr-selleck.pdf
WO2014035064A1...Figure 7 is the 1H nuclear magnetic resonance (NMR) spectrum of tenofovir disoproxil fumarate
PAPER
Volume 107, 25 March 2015, Pages 175–185
Isolation, LC–MS/MS and 2D-NMR characterization of alkaline degradants of tenofovir disoproxil fumarate
- a Department of Pharmaceutical Analysis, National Institute of Pharmaceutical Education and Research (NIPER), Balanagar, Hyderabad 500037, India
- b Analytical Chemistry Division, Daiichi Sankyo Life Sciences Research Centre in India (RCI), Gurgaon 122015, India
PAPER

The current three-step manufacturing route for the preparation of tenofovir disoproxil fumarate (1)
was assessed and optimized leading to a higher yielding, simpler, and
greener process. Key improvements in the process route include the
refinement of the second stage through the replacement of the
problematic magnesium tert-butoxide (MTB) with a 1:1 ratio of a Grignard reagent and tert-butanol.
The development of a virtually solvent-free approach and the
establishment of a workup and purification protocol which allows the
isolation of a pure diethyl phosphonate ester (8) was achieved

see.............http://pubs.acs.org/doi/abs/10.1021/acs.oprd.5b00364
An Improved Process for the Preparation of Tenofovir Disoproxil Fumarate
† Department of Chemistry, Natural and Agricultural Sciences, University of Pretoria, 2 Lynnwood Road, Hatfield, 0002, Gauteng, South Africa
‡ Department of Engineering and Technology Management, University of Pretoria, Pretoria, South Africa
§ Pharmaceutical Manufacturing Technology Centre, University of Limerick, Limerick, V94 T9PX, Republic of Ireland
∥ iThemba Pharmaceuticals, Modderfontein, 1645, Gauteng South Africa
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.5b00364
Publication Date (Web): March 04, 2016
Copyright © 2016 American Chemical Society
*E-mail: darren.riley@up.ac.za.

Department of Chemistry, Natural and Agricultural Sciences, University of Pretoria, 2 Lynnwood Road, Hatfield, 0002, Gauteng, South Africa

PAPER........http://www.hindawi.com/journals/jchem/2013/126502/
Journal of Chemistry
Volume 2013 (2013), Article ID 126502, 12 pages
http://dx.doi.org/10.1155/2013/126502
Research Article
Vibrational Spectroscopic Studies of Tenofovir Using Density Functional Theory Method
G. R. Ramkumaar,1 S. Srinivasan,2 T. J. Bhoopathy,1 and S. Gunasekaran1
1PG and Research Department of Physics, Pachaiyappa's College, Chennai 600030, India
2PG and Research Department of Physics, Presidency College, Chennai 600005, India
Figure 3: Theoretical and experimental IR spectrum of tenofovir.
T able 6: The calculated 13C and 1H NMR chemical shifts of tenofovir.
Atom position B3LYP/6-311++ B3LYP/6-31 B3PW91/6-31 ChemDraw Ultra Assignment
Absolute shielding Chemical shift Absolute shielding Chemical shift Absolute shielding Chemical shift
1 114.7 85.3 126.6 73.3 129.7 70.3 73.4 C1 in Aliphatic
3 124.7 75.3 136.6 63.4 139.3 60.7 58.6 C3 in Aliphatic
4 122.5 77.4 134.7 65.3 137.5 62.4 76 C4 in Aliphatic
5 153.9 46.1 163.3 36.7 166.4 33.6 20 C5 in Aliphatic
7 36.4 163.5 56.7 143.3 58.8 141.1 143 C7 in Purine
9 25.3 174.7 44.9 155.1 47.3 152.6 149.8 C9 in Purine
11 23.0 177.0 42.9 157.1 45.1 154.9 152.4 C11 in Purine
13 33.6 166.4 55.0 144.9 56.9 143.1 156.1 C13 in Purine
14 62.8 137.2 81.3 118.6 83.6 116.4 119.4 C14 in Purine
20 27.8 4.8 28.0 4.6 27.9 4.7 3.4 H20 in Methylene
21 27.9 4.7 28.0 4.6 28.0 4.6 3.4 H21 in Methylene
22 27.5 5.1 27.5 5.1 27.4 5.2 3.9 H22 in Methylene
23 26.8 5.8 26.7 5.9 26.6 6.0 3.7 H23 in Methyene
24 16.4 16.2 16.3 16.3 16.2 16.4 12.0 H24 in Methine
25 29.7 2.9 29.7 2.9 29.6 3.0 1.2 H25 in Methyl
26 30.3 2.3 30.2 2.4 30.2 2.4 1.2 H26 in Methyl
27 30.3 2.3 30.3 2.3 30.2 2.4 1.2 H27 in Methyl
28 23.8 8.8 23.8 8.8 23.6 9.0 8.1 H28 in Purine
29 23.3 9.3 23.1 9.5 22.9 9.7 8.2 H29 in Purine
30 25.0 7.6 25.2 7.4 25.0 7.6 7.0 H30 in Aromatic
31 25.6 7.0 25.9 6.7 25.7 6.9 7.0 H31 in Aromatic
32 29.2 3.4 29.0 3.6 28.9 3.7 12.0 H32 in Alcohol
33 28.3 4.3 28.2 4.4 28.1 4.5 12.0 H33 in Alcohol
(a)
(b)
Figure 5: (a) 13C NMR spectrum of tenofovir. (b) 1H NMR spectrum of tenofovir.
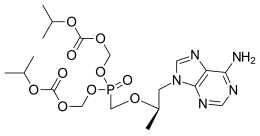 |
|
| Systematic (IUPAC) name | |
|---|---|
Bis{[(isopropoxycarbonyl)oxy]methyl} ({[(2R)-1-(6-amino-9H-purin-9-yl)-2-propanyl]oxy}methyl)phosphonate
|
|
| Clinical data | |
| Trade names | Viread |
| AHFS/Drugs.com | monograph |
| Pregnancy category |
|
| Routes of administration |
Oral (tablets) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 25% |
| Identifiers | |
| CAS Number | 201341-05-1 |
| ATC code | J05AF07 (WHO) |
| PubChem | CID 5481350 |
| ChemSpider | 4587262 |
| UNII | F4YU4LON7I |
| ChEBI | CHEBI:63717 |
| NIAID ChemDB | 080741 |
| Chemical data | |
| Formula | C19H30N5O10P |
| Molar mass | 519.443 g/mol |
 |
|
| Systematic (IUPAC) name | |
|---|---|
({[(2R)-1-(6-amino-9H-purin-9-yl)propan-2-yl]oxy}methyl)phosphonic acid
|
|
| Clinical data | |
| MedlinePlus | a602018 |
| Routes of administration |
In form of prodrugs |
| Pharmacokinetic data | |
| Protein binding | < 1% |
| Biological half-life | 17 hours |
| Excretion | Renal |
| Identifiers | |
| CAS Number | 147127-20-6 |
| ATC code | None |
| PubChem | CID 464205 |
| DrugBank | DB00300 |
| ChemSpider | 408154 |
| UNII | 99YXE507IL |
| KEGG | D06074 |
| ChEBI | CHEBI:63625 |
| ChEMBL | CHEMBL483 |
| Synonyms | 9-(2-Phosphonyl-methoxypropyly)adenine (PMPA) |
| Chemical data | |
| Formula | C9H14N5O4P |
| Molar mass | 287.213 g/mol |
SEE........ http://www.ijcps.com/files/vol3issue3/17.pdf
http://shodhganga.inflibnet.ac.in/bitstream/10603/18837/5/05_chapter%203.pdf
///////