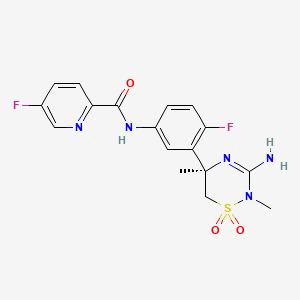
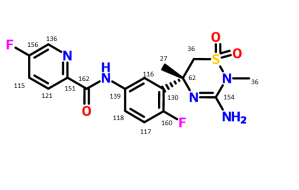
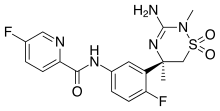
Verubecestat (MK-8931)
Merck Alzheimer's drugs Verubecestat (MK-8931) is an oral β- amyloid precursor protein cleaving enzyme (BACE1 or β-secretase enzyme) inhibitor, is currently in Phase III clinical trials
Verubecestat
MK 8931, MK-8931, SCH 900931
2-Pyridinecarboxamide, N- (3 - ((5R) -3-amino-5,6-dihydro-2,5-dimethyl-1 , 1-dioxido-2H-1,2,4-thiadiazin-5-yl) -4-fluorophenyl) -5-fluoro-
N-[3-[(5R)-3-amino-2,5-dimethyl-1,1-dioxo-6H-1,2,4-thiadiazin-5-yl]-4-fluorophenyl]-5-fluoropyridine-2-carboxamide
CAS : 1286770-55-5
Mechanism: Oral β- amyloid precursor protein cleavage enzyme (BACE) inhibitors
Indications: Alzheimer's disease
Development progress: phase III clinical
Companies: Merck
Verubecestat (MK-8931) is a small-molecule inhibitor of beta-secretase cleaving enzyme (BACE) 1 and BACE2 in development by Merck for the treatment of Alzheimer's Disease.
MK-8931 is a beta-secretase 1 (BACE1) inhibitor in phase III development for the treatment of amnestic mild cognitive impairment (aMCI) due to Alzheimer's disease at Merck & Co. The company is also conducting phase II/III trials for the treatment of Alzheimer's type dementia.
Smiles: C [C @] 1 (CS (= O) (= O) N (C (= N1) N) C) c2cc (ccc2F) NC (= O) c3ccc (cn3) F
COSY PREDICT
 https://www.google.co.in/patents/CN102639135A?cl=en
Scheme 3b:
The amine A (Scheme 3a, step 4) (13.7 g) in n-butanol (150 mL) was added a slurry solution of cyanogen bromide (5M, in MeCN). The resulting mixture was heated to reflux for 4 hours. The mixture was concentrated to 1/3 of original volume. To this mixture was added Et20 (200 mL). The resulting solid was removed by filtration, and the solid was washed with Et20 (2x). The solid was partitioned between EtOAc and saturated Na2CO3 (aq). The aqueous layer was extracted with EtOAc (3x). The combined organic layers were washed with brine, dried over Na2SO4, filtered and concentrated to give 10.6 g
Scheme 10:
The nitro compound (Scheme 3b) (2. 50 g, 6. 0 mmol) of Et0H (150 mL) was degassed (To this solution was bubbled with nitrogen time 3 min). To this solution was added Pd / C (10% w / w, 50% water, 698 mg). The mixture was placed in a nitrogen atmosphere. Exhaust, and backfilled with H2 (3x). The obtained mixture at room temperature, followed by stirring under H2 balloon for 2 hours. Bubbling nitrogen gas, and the mixture was purged, filtered through Celite, and concentrated.Small plug filtered through a silica gel column, eluting with EtOAc, and the product was purified to give the aniline (2. 2g, 97%).
https://www.google.co.in/patents/CN102639135A?cl=en
Scheme 3b:
The amine A (Scheme 3a, step 4) (13.7 g) in n-butanol (150 mL) was added a slurry solution of cyanogen bromide (5M, in MeCN). The resulting mixture was heated to reflux for 4 hours. The mixture was concentrated to 1/3 of original volume. To this mixture was added Et20 (200 mL). The resulting solid was removed by filtration, and the solid was washed with Et20 (2x). The solid was partitioned between EtOAc and saturated Na2CO3 (aq). The aqueous layer was extracted with EtOAc (3x). The combined organic layers were washed with brine, dried over Na2SO4, filtered and concentrated to give 10.6 g
Scheme 10:
The nitro compound (Scheme 3b) (2. 50 g, 6. 0 mmol) of Et0H (150 mL) was degassed (To this solution was bubbled with nitrogen time 3 min). To this solution was added Pd / C (10% w / w, 50% water, 698 mg). The mixture was placed in a nitrogen atmosphere. Exhaust, and backfilled with H2 (3x). The obtained mixture at room temperature, followed by stirring under H2 balloon for 2 hours. Bubbling nitrogen gas, and the mixture was purged, filtered through Celite, and concentrated.Small plug filtered through a silica gel column, eluting with EtOAc, and the product was purified to give the aniline (2. 2g, 97%).
 SEE
PATENT
http://www.google.co.in/patents/WO2011044181A1?cl=en
SEE
PATENT
http://www.google.co.in/patents/WO2011044181A1?cl=en
 SNAPSHOT
SNAPSHOT
 SYNTHESIS CONSTRUCTION
SYNTHESIS CONSTRUCTION

AND

ON RXN WITH WITH BuLi GIVES
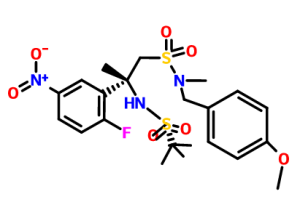
THIS GIVES
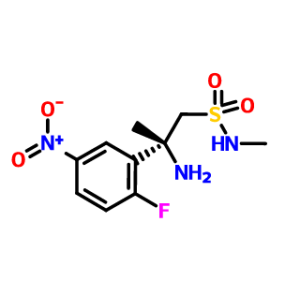
THIS ON TREATMENT WITH BrCN
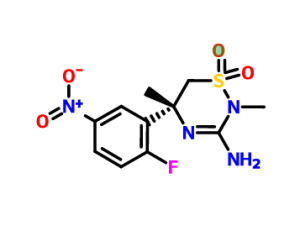
ON BOC2O TREATMENT GIVES
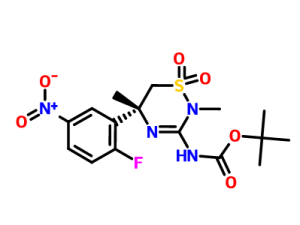
GIVES ON HYDGN
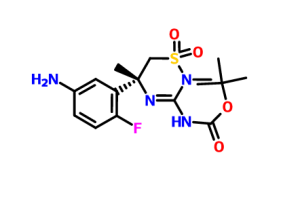
REACTION WITH

GIVES
FINAL COMPD Verubecestat
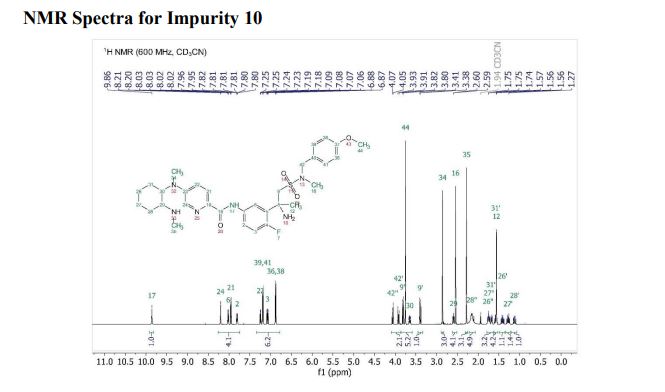
 1H NMR PREDICT
1H NMR PREDICT
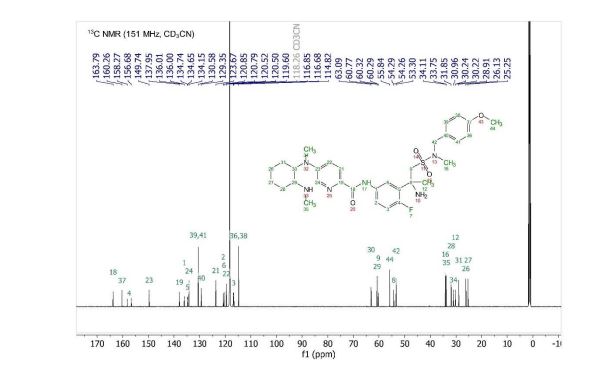
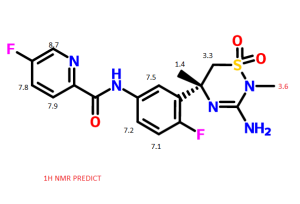 13C NMR PREDICT
13C NMR PREDICT
Updated.......WATCH OUT FOR MORE
https://www.google.co.in/patents/US8729071?cl=en

Steps 1-4:
These steps were performed using similar procedures to those described in steps 1-4 of Scheme 1a.
Step 5:
To a solution of the amine from step 4 (10.5 g, 36 mmol) in CH
2Cl
2 (200
mL) was added benzoylisothiocyanate (4.3 mL, 1.1 eq.). The resulting
solution was stirred at RT for 2.5 days. Additional
benzoylisothiocyanate (0.86 mL, 0.2 eq.) was added and the solution was
stirred at RT for an additional 2 hours. The solution was then
concentrated in vacuo.
A portion of this material (6.5 g, ˜14 mmol) was dissolved in MeOH (200 mL). To this solution was added Na
2CO
3 (s) (1.52
g, 14 mmol). The resultant mixture was stirred at RT for 45 min. After
that time, a slight excess of HOAc was added to the solution. The
mixture was then concentrated. The residue was partitioned between CH
2Cl
2 and ½ sat. NaHCO
3 (aq.). The aqueous layer was extracted with CH
2Cl
2 (3×). The combined organic layers were dried over Na
2SO
4, filtered and concentrated. The thiourea (˜4.9 g) was carried onto the next reaction without further purification.
Step 6:
Example 15 was prepared using a method similar to that described in Scheme 1a step 6.
To
a shiny of amine A (Scheme 3a step 4) (13.7 grams) in n-butanol (150
mL) was added a solution of cyanogen bromide (5M in MeCN). The resultant
mixture was heated to reflux for 4 hours. The mixture was concentrated
to ⅓ of the original volume. To the mixture was added Et
2O (200 mL). The resultant solid was removed via filtration and the solid was washed with Et
2O (2×). The solid was partitioned between EtOAc and sat. Na
2CO
3 (aq.). The aqueous layer was extracted with EtOAc (3×). The combined organic layers were washed with brine, dried over Na
2SO
4,
filtered and concentrated to afford 10.6 grams of Ex. 15. This material
was converted to the t-butyl carbamate using a procedure similar to
that described in Scheme 3.
Step 7:
A mixture of the bromide (3.00 g, 6.92 mmol), benzophenone imine (1.39 mL, 8.30 mmol), Pd
2(dba)
3 (0.634
g, 0.692 mmol), John-Phos (0.413 g, 1.38 mmol), sodium tert-butoxide
(2.13 g, 22.1 mmol), and toluene (51 mL) was degassed (vacuum/N
2).
The mixture was then stirred at 65° C. under nitrogen for 3 h. After
this time, the reaction mixture was cooled to room temperature and
filtered through a pad of Celite and rinsed with ethyl acetate (100 mL).
The filtrate was concentrated under reduced pressure. The residue was
then dissolved in methanol (76 mL) and the resulting solution was
charged with hydroxyl amine hydrochloride (2.16 g, 31.1 mmol) and sodium
acetate (2.55 g, 31.1 mmol). The reaction mixture was stirred at room
temperature for 40 min. After this time, the reaction mixture was
concentrated under reduced pressure. The resulting residue was dissolved
in ethyl acetate (200 mL) and washed with saturated aqueous sodium
bicarbonate (100 mL), water (100 mL), and brine (100 mL). The organic
layer was then dried over anhydrous sodium sulfate, filtered, and
concentrated under reduced pressure. The residue was purified by column
chromatography (silica, 0-100% ethyl acetate/heptane) to afford the
amino pyridine (0.880 g, 34%).

To a flame-dried flask was added a pyridyl bromide (Table IIb, Entry 15, 1.5 g, 3.3 mmol), Pd
2(dba)
3 (305
mg, 0.3 mmol), (2-biphenyl)di-tert-butylphosphine (200 mg, 0.7 mmol),
sodium tert-butoxide (1.02 g, 0.011 mmol), benzophenone imine (670 ul, 4
mmol), and toluene (21 mL). The mixture was evacuated under vacuum and
back-filled with N
2 (3×). The mixture was stirred at 60° C.
for 1 h. After filtration through celite, the filtrate was concentrated.
The crude residue was dissolved in 36 mL of methanol, and hydroxyl
amine hydrochloride (458 mg, 6.6 mmol) and sodium acetate (541 mg, 6.6
mmol) were added. The reaction was stirred for 35 min and then quenched
with saturated aqueous sodium bicarbonate. The mixture was extracted
with ethyl acetate, and the combined organic portions were dried over
magnesium sulfate and concentrated. The crude residue was purified by a
flash silica column (50% ethyl acetate/hexane) to get an aminopyridine
product (730 mg, 68%).
A solution of the nitro compound (Scheme 3b) (2.50 g, 6.0 mmol) in EtOH (150 mL) was degassed by bubbling N
2 through the solution for 3 min. To this solution was added Pd/C (10% w/w, 50% H
2O, 698 mg.). The mixture was placed under an atmosphere of N
2. The atmosphere was evacuated and back-filled with H
2 (3×). The resulting mixture was stirred at RT under a H
2 balloon for 2 h. The mixture was purged by bubbling N
2 through
it, filtered through Celite and concentrated. The product was purified
by filtering through a small plug of silica gel column eluting with
EtOAc to afford the aniline (2.2 g, 97%).
| ENTRY 25 |
MH+: 410.0, HPLC1.79 min, LCMSMETHOD D |
Method D:
- Column: Agilent Zorbax SB-C18 (3.0×50 mm) 1.8 uM
Mobile phase: A: 0.05% Trifluoroacetic acid in water
- B: 0.05% Trifluoroacetic acid in acetonitrile
Gradient: 90:10 (A:B) for 0.3 min, 90:10 to 5:95 (A:B) over 1.2 min, 5:95 (A:B) for 1.2 min.
Flow rate: 1.0 mL/min
UV detection: 254 and 220 nm
Mass spectrometer: Agilent 6140 quadrupole
सुकून उतना ही देना प्रभू, जितने से जिंदगी चल जाये। औकात बस इतनी देना, कि औरों का भला हो जाये। DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
Join me on Linkedin

Join me on Facebook
 FACEBOOK
FACEBOOK
Join me on twitter

 amcrasto@gmail.com
amcrasto@gmail.com

LIONEL MY SON
He was only in first standard in school when I was hit by a deadly one in a million spine stroke called acute transverse mylitis, it made me 90% paralysed and bound to a wheel chair, Now I keep him as my source of inspiration and helping millions, thanks to millions of my readers who keep me going and help me to keep my son happy
सुकून उतना ही देना प्रभू, जितने से
जिंदगी चल जाये।
औकात बस इतनी देना,
कि औरों का भला हो जाये।
///////




























 LIONEL MY SON
LIONEL MY SON