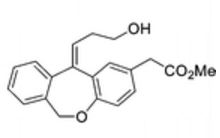
E/Z
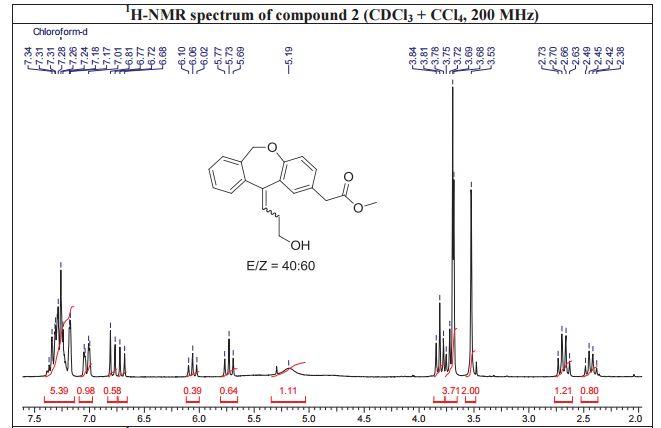
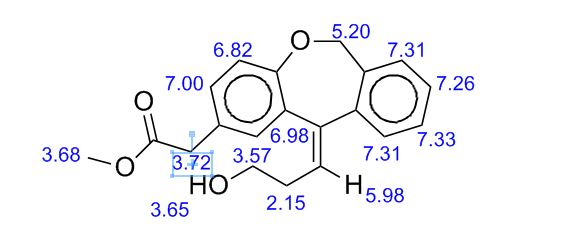
White solid; Ή NMR (200 MHz, CDC13 + CC14): δ 2.38-2.49 ( m,0.8H, E- Form), 2.63-2.73 ( m,1.2H, Z-Form), 3.53 (s, 2H), 3.68 (s, 3H), 3.75 (m, 0.8H, E-Form), 3.81 (t, J=6.3 Hz, 1.2H), 5.19 (brs, 2H), 5.73 (t, J=7.8 Hz, 0.6H, Z-Form), 6.06 (t, J=7.8 Hz, 0.4H, E-Form), 6.70 (d, J=8.2 Hz, 0.4 H, E-Form), 6.79 (d, J=8.2 Hz, 0.6 H, Z- Form), 7.00-7.34 (m, 6H), HRMS m/r. Calculated for C20H2,O4-325.1434, observed- 325.1437.
CLIP 2
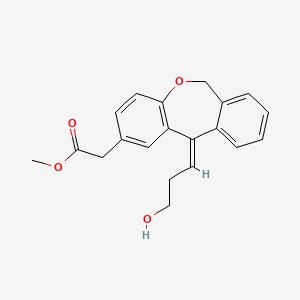
Methyl (Z)-11-[(3-Hydroxy)propylidene]-6,11-dihydrobenz[b,e]oxepin-2-acetate
916243-39-5 cas
mf C20 H20 O4
Dibenz[b,e]oxepin-2-acetic acid, 6,11-dihydro-11-(3-hydroxypropylidene)-, methyl ester, (11Z)-
- Molecular Weight, 324.37
white colorless crystal;
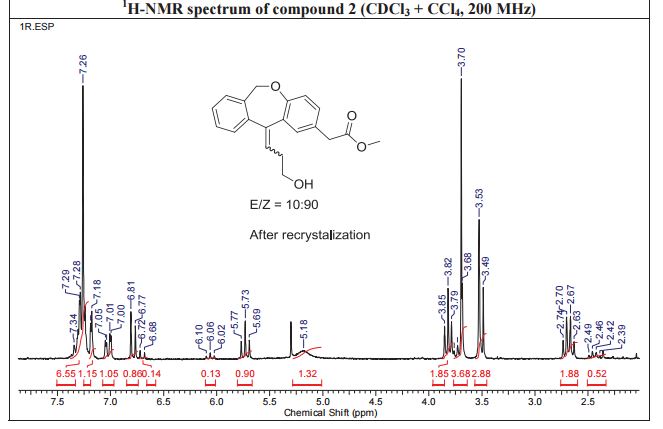
1H NMR (CDCl3, 300 MHz) δ 7.34–7.23 (m, 4H), 7.17 (d, J = 2.2 Hz, 1H), 7.04 (dd, J = 8.4, 2.2 Hz, 1H), 6.80 (d, J = 8.4 Hz, 1H), 5.74 (t, J = 7.5 Hz, 1H), 5.18 (brs, 2H), 3.80 (t, J = 6.1 Hz, 2H), 3.69 (s, 3H), 3.53 (s, 2H), 2.68 (dt, J = 7.5, 6.1 Hz, 2H);
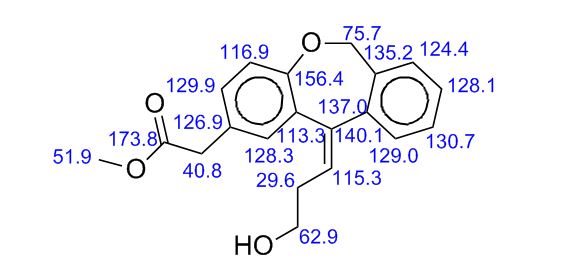
13C NMR (CDCl3, 75 MHz): δ 172.4, 154.6, 145.3, 141.4, 133.6, 132.1, 130.0, 129.1, 127.5, 126.2, 125.7, 124.0, 119.7, 70.5, 62.6, 52.1, 40.1, 33.3;
MS ESI (+) m/z 325 [M + H]+.
Org. Process Res. Dev., 2012, 16 (2), pp 225–231
DOI: 10.1021/op200312m
CLIP 3
Synthesis 2013; 45(24): 3399-3403
DOI: 10.1055/s-0033-1340008
DOI: 10.1055/s-0033-1340008
CLICK ON IMAGE
1H AND 13C NMR PREDICT
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This article is a compilation for educational purposes only.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
//////O=C(OC)Cc1ccc2OCc3ccccc3C(=C\CCO)\c2c1