Aplaviroc
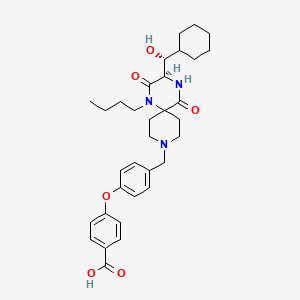
4-(4-{[(3R)-1-butyl-3-[(R)-cyclohexylhydroxymethyl]-2,5-dioxo- 1,4,9-triazaspiro[5.5]undecan-9-yl]methyl}phenoxy)benzoic acid
for the treatment of HIV infection
461023-63-2 of hydrochloride
461443-59-4 (free base)
873140
AK-602
GW-873140
ONO-4128
AK-602
GW-873140
ONO-4128
ono…….innovator
| Ono Pharmaceutical Co., Ltd. |
Base
4-[4-[[(3R)-1-Butyl-3-[(R)-cyclohexylhydroxymethyl]-2,5-dioxo-1,4,9-triazaspiro[5.5]undec-9-yl]methyl]phenoxy]benzoic acid
(3R)-1-butyl-2,5-dioxo-3-[(1R)-1-hydroxy-1-cyclohexylmethyl]-9-[4-(4-carboxyphenyloxy)phenylmethyl]-1,4,9-triazaspiro[5.5]undecane
Molecular Formula: C33H43N3O6
Molecular Weight: 577.71
Percent Composition: C 68.61%, H 7.50%, N 7.27%, O 16.62%
References: CCR5 chemokine receptor antagonist; inhibits HIV entry by blocking interaction of viral coat protein gp120 with the receptor. Prepn: H. Habashita et al., WO02074770 (2002 to Ono); eidem, US 04082584 (2004).
Study of CCR5 binding and mechanism of action: C. Watson et al., Mol. Pharmacol. 67, 1268 (2005).
Antiretroviral activity in immunodeficient mice: H. Nakata et al., J. Virol. 79, 2087 (2005). Clinical pharmacokinetics: K. K. Adkison et al., Antimicrob. Agents Chemother. 49, 2802 (2005).
Derivative Type: Hydrochloride
CAS Registry Number: 461023-63-2
Manufacturers’ Codes: AK-602; GW-873140; ONO-4128
Molecular Formula: C33H43N3O6.HCl
Molecular Weight: 614.17
Percent Composition: C 64.53%, H 7.22%, N 6.84%, O 15.63%, Cl 5.77%
Therap-Cat: Antiviral.
| IDENTIFIERS | |
|---|---|
| CAS NUMBER | 461023-63-2 |
| ATC CODE | None |
| PUBCHEM | CID 3001322 |
| CHEMSPIDER | 2272720 |
| UNII | 98B425P30V |
| KEGG | D06557 |
| CHEMBL | CHEMBL1255794 |
| CHEMICAL DATA | |
| FORMULA | C33H43N3O6 |
| MOL. MASS | 577.711 g/mol |
Aplaviroc (INN, codenamed AK602 and GSK-873140) is a CCR5 entry inhibitor developed for the treatment of HIV infection.[1][2] It is developed by GlaxoSmithKline.
In October 2005, all studies of aplaviroc were discontinued due to liver toxicity concerns.[3][4] Some authors have claimed that evidence of poor efficacy may have contributed to termination of the drug’s development;[5] the ASCENT study, one of the discontinued trials, showed aplaviroc to be under-effective in many patients even at high concentrations.[6]
Aplaviroc hydrochloride, an orally-effective, long-acting chemokine CCR5 receptor antagonist, had been under development by Ono and GlaxoSmithKline for the treatment of HIV infection. In early 2006, the companies discontinued development of the antagonist based on reports of elevated liver function test values from clinical studies.
Originally developed at Ono, aplaviroc was licensed to GlaxoSmithKline in 2003 for development, manufacturing and marketing. GlaxoSmithKline also obtained rights to evaluate the agent in non-HIV conditions worldwide with the exception of Japan, South Korea and Taiwan.
A low-molecular-weight compound, aplaviroc prevents HIV viral infection by blocking the binding of the virus to the CCR5 receptor
……………….
WO 2002074770
0r
Example 9(54)
- (3R)-1-butyl-2,5-dioxo-3-((1R)-1-hydroxy-1-cyclohexylmethyl)-9-(4-(4-carboxyphenyloxy)phenylmethyl)-1,4,9-triazaspiro[5.5]undecane • hydrochloride
- [0359]TLC:Rf 0.43(chloroform:methanol = 5:1);
NMR (CD3OD):δ 8.05 (d, J = 9.0 Hz, 2H), 7.61 (d, J = 9.0 Hz, 2H), 7.19 (d, J = 9.0 Hz, 2H), 7.08 (d, J = 9.0 Hz, 2H), 4.38 (s, 2H), 4.17 (d, J = 2.1 Hz, 1H), 4.02 (m, 1H), 3.78 (m, 1H), 3.60-3.40 (m, 3H), 3.30-3.10 (m, 2H), 2.56-1.86 (m, 6H), 1.82-1.60 (m, 5H), 1.52-1.16 (m, 6H), 1.06-0.82 (m, 2H), 0.97 (t, J = 7.2 Hz, 3H).
read at
http://newdrugapprovals.org/2014/11/19/aplaviroc-ak602-gsk-873140/