A borrowing hydrogen methodology: palladium-catalyzed dehydrative N-benzylation of 2-aminopyridines in water
Abstract
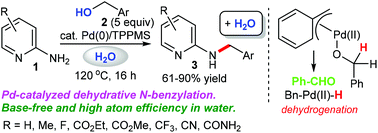
We demonstrate a greener borrowing hydrogen methodology using the π-benzylpalladium system, which offers an efficient and environmentally friendly dehydrative N-monobenzylation of 2-aminopyridines with benzylic alcohols in the absence of base. The crossover experiment using benzyl-α,α-d2 alcohol and 3-methylbenzyl alcohol afforded H/D scrambled products, suggesting that the dehydrative N-benzylation in our catalytic system involves a borrowing hydrogen pathway. KIE experiments show that C–H bond cleavage at the benzylic position of benzyl alcohol is involved in the rate-determining step (KIE = 2.9). This simple base-free protocol can be achieved under mild conditions in an atom-economic process, affording the desired products in moderate to excellent yields.
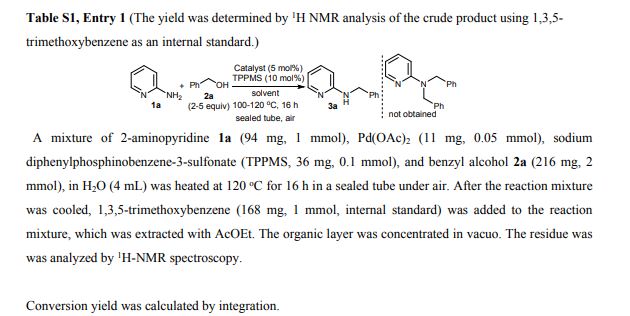
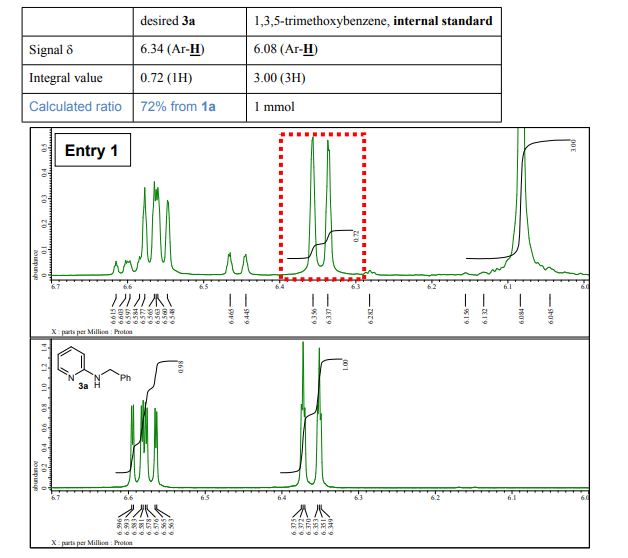
N-Benzylpyridin-2-amine 3a 1 Yield 165 mg (90%) as a white solid; mp 90-91 C; IR (KBr) (cm-1) 3226, 3029, 1600, 1575; 1H NMR (400 MHz, CDCl3): 4.50 (d, J=5.7 Hz, 2H), 4.95 (brs, 1H), 6.36 (dt, J=8.5, 0.9 Hz, 1H), 6.58 (ddd, J=7.1, 5.0, 0.9 Hz, 1H), 7.23-7.36 (m, 4H), 7.39 (dd, J=8.7, 7.1, 1.8 Hz, 1H), 8.09 (ddd, J=5.0, 1.8, 0.9 Hz, 2H); 13C-NMR (100 MHz, CDCl3): 46.3, 106.8, 113.1, 127.2, 127.4, 128.6, 137.5, 139.2, 148.2, 158.6; MS (FAB): m/z 185 [M+H]+ .


/////////////borrowing hydrogen methodology, palladium-catalyzed, dehydrative N-benzylation, 2-aminopyridines,N-Benzylpyridin-2-amine 3a 1 Yield 165 mg (90%) as a white solid; mp 90-91 C; IR (KBr) (cm-1) 3226, 3029, 1600, 1575; 1H NMR (400 MHz, CDCl3): 4.50 (d, J=5.7 Hz, 2H), 4.95 (brs, 1H), 6.36 (dt, J=8.5, 0.9 Hz, 1H), 6.58 (ddd, J=7.1, 5.0, 0.9 Hz, 1H), 7.23-7.36 (m, 4H), 7.39 (dd, J=8.7, 7.1, 1.8 Hz, 1H), 8.09 (ddd, J=5.0, 1.8, 0.9 Hz, 2H); 13C-NMR (100 MHz, CDCl3): 46.3, 106.8, 113.1, 127.2, 127.4, 128.6, 137.5, 139.2, 148.2, 158.6; MS (FAB): m/z 185 [M+H]+ .