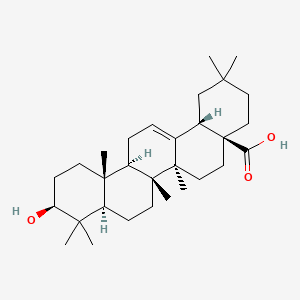
 Oleanolic acid
(4aS,6aR,6aS,6bR,8aR,10S,12aR,14bS)-10-hydroxy-2,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid
Oleanolic acid
(4aS,6aR,6aS,6bR,8aR,10S,12aR,14bS)-10-hydroxy-2,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid
Oleanic acid, Caryophyllin, Astrantiagenin C, Giganteumgenin C, Virgaureagenin B, 3beta-Hydroxyolean-12-en-28-oic acid, OLEANOLIC_ACID
Molecular Formula: C30H48O3
Molecular Weight: 456.70032
 Ursolic acid [(3b)-3-Hydroxyurs-12-en-28-oic acid] rarely occurs without its isomer oleanolic acid [(3b)-3-Hydroxyolean-12-en-28-oic acid] They may occur in their free acid form, as shown in Figure 1, or as aglycones for triterpenoid saponins which are comprised of a triterpenoid aglycone linked to one or more sugar moieties. Ursolic and oleanolic acids are similar in pharmacological activity
A pentacyclic triterpene that occurs widely in many PLANTS as the free acid or the aglycone for many SAPONINS. It is biosynthesized from lupane. It can rearrange to the isomer, ursolic acid, or be oxidized to taraxasterol and amyrin.
Ursolic acid [(3b)-3-Hydroxyurs-12-en-28-oic acid] rarely occurs without its isomer oleanolic acid [(3b)-3-Hydroxyolean-12-en-28-oic acid] They may occur in their free acid form, as shown in Figure 1, or as aglycones for triterpenoid saponins which are comprised of a triterpenoid aglycone linked to one or more sugar moieties. Ursolic and oleanolic acids are similar in pharmacological activity
A pentacyclic triterpene that occurs widely in many PLANTS as the free acid or the aglycone for many SAPONINS. It is biosynthesized from lupane. It can rearrange to the isomer, ursolic acid, or be oxidized to taraxasterol and amyrin.
MS
EIMS m/z (rel. int.) 456 [M]+ (5), 412 (3), 248 (100), 203 (50), 167 (25), 44 (51)
IR KBR
(KBr) 3500, 2950, 2850, 1715; 1H-NMR (250 MHz, pyridine-d5) δ: 5.49 (1H, s, H-12), 3.47 (1H, t, J = 8.0 Hz, H-3), 3.30 (1H, m, H-18), 1.12 (3H, s, CH3-27), 0.96 (3H, s, CH3-30), 0.91 (3H, s, CH3-25), 0.89 (3H, s, CH3-23), 0.87 (3H, s, CH3-24), 0.75 (3H, s, CH3-26)
1H NMR
|
(250 MHz, pyridine-d5)
δ: 5.49 (1H, s, H-12), 3.47 (1H, t, J = 8.0 Hz, H-3), 3.30 (1H, m, H-18), 1.12 (3H, s, CH3-27), 0.96 (3H, s, CH3-30), 0.91 (3H, s, CH3-25), 0.89 (3H, s, CH3-23), 0.87 (3H, s, CH3-24), 0.75 (3H, s, CH3-26)

|

13 C NMR
|
(63 MHz, pyridine-d5) δ: 180.2 (C-28), 144.8 (C-13), 122.5 (C-12), 78.0 (C-3), 55.7 (C-5), 48.0 (C-9), 46.6 (C-8, 17), 42.1 (C-14), 39.7 (C-4), 39.4 (C-1), 37.3 (C-10), 33.2 (C-7), 32.9 (C-29), 32.4 (C-21), 30.9 (C-20), 28.7 (C-23), 27.2 (C-2), 26.9 (C-15), 26.1 (C-30), 23.7 (C-11), 23.6 (C-16), 18.7 (C-6), 17.4 (C-26), 16.5 (C-24), 15.5 (C-25)

|
 http://www.google.com/patents/US20120237629
http://www.google.com/patents/US20120237629
FIG. 4 shows the 1H NMR spectrum of oleanolic acid;
FIG. 5 shows the 13C NMR spectrum of oleanolic acid;
FIG. 6 shows the 13C DEPT NMR spectrum of oleanolic acid;
FIG. 7 shows the 1H 13C HSQC NMR spectrum of oleanolic acid;
see below



 EXAMPLE 2 Extraction and Isolation of Oleanolic Acid (9) and Maslinic Acid (10) from Cloves
EXAMPLE 2 Extraction and Isolation of Oleanolic Acid (9) and Maslinic Acid (10) from Cloves
Syzygium aromaticum dried buds or whole cloves were obtained commercially. The cloves (1.5 kg, whole) of Syzygium aromaticum were sequentially and exhaustively extracted with hexane and ethyl acetate to give, after solvent removal in vacuo, a hexane extract (68.8 g, 4.9%) and an ethyl acetate extract (34.1 g, 2.3%). A portion of the ethyl acetate extract (10.0 g), was subjected to chromatographic separation on silica gel (60-120 mesh) column (40×5.0 cm). Elution with hexane/ethyl acetate solvent mixtures (8:2→6:4) afforded pure oleanolic acid (9) (4.7 g, 1.06%), a mixture of oleanolic acid (9) and maslinic acid (10) (0.5 g), and pure maslinic acid (10) (0.25 g). The structures of oleanolic acid (9) and maslinic acid (10) (as 2,3-diacetoxyoleanolic acid) were confirmed by spectroscopic data analysis (1D and 2D 1H NMR and 13C NMR experiments) (FIGS. 4-7 and FIGS. 8-10, respectively).
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE