A fully continuous-flow diazotization–hydrolysis protocol has been developed for the preparation of p-cresol. This process started from the diazotization of p-toluidine to form diazonium intermediate. The reaction was then quenched by urea and subsequently followed by a hydrolysis to give the final product p-cresol. Three types of byproducts were initially found in this reaction sequence. After an optimization of reaction conditions (based on impurity analysis), side reactions were eminently inhibited, and a total yield up to 91% were ultimately obtained with a productivity of 388 g/h. The continuous-flow methodology was used to avoid accumulation of the highly energetic and potentially explosive diazonium salt to realize the safe preparation for p-cresol.
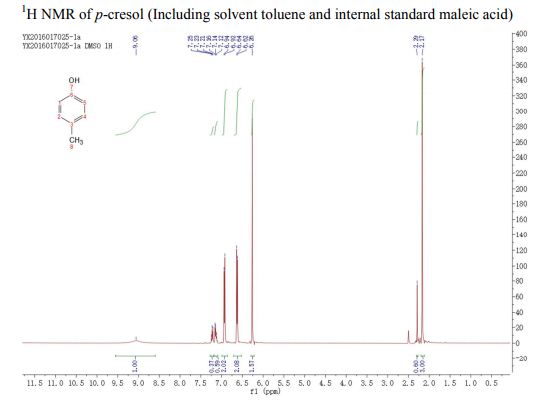
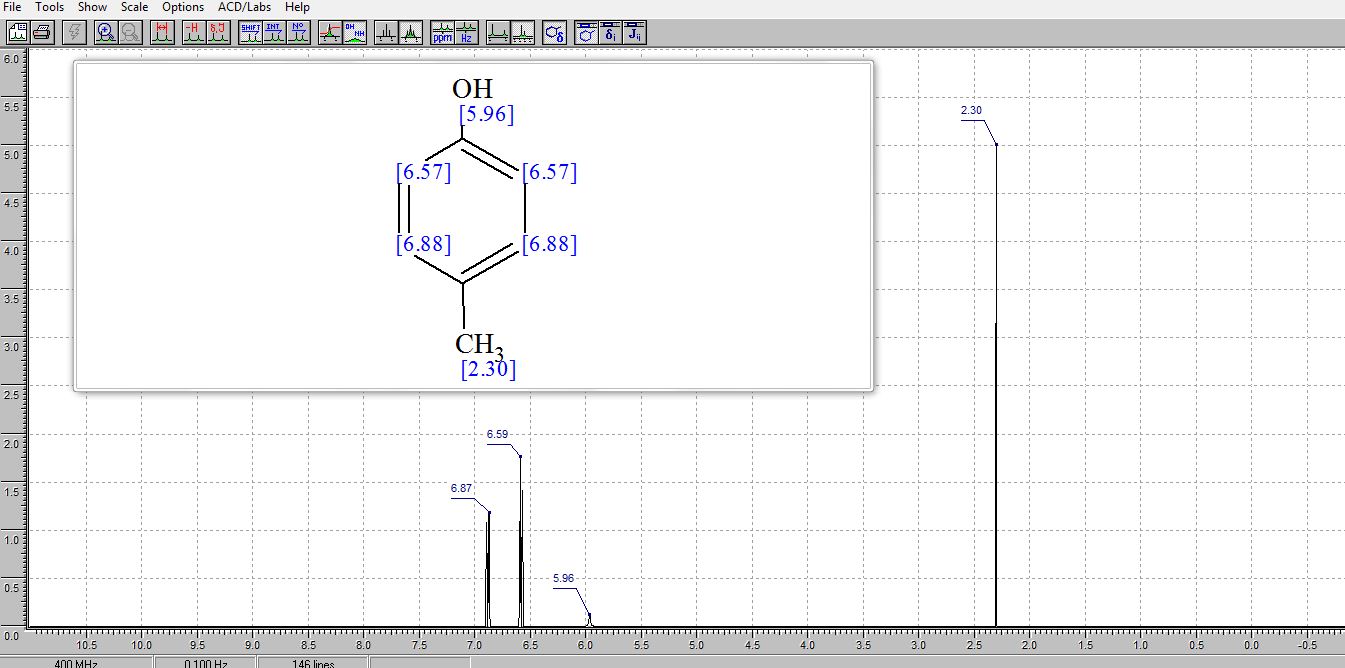
. 1H NMR (400 MHz, (CD3)2SO) δ/ppm: 9.06 (br s, 1H, −OH), 6.94 (d, J = 8.0 Hz, 2H, Ar–H), 6.62 (d, J = 8.0 Hz, 2H, Ar–H), 2.17 (s, 3H, −CH3).
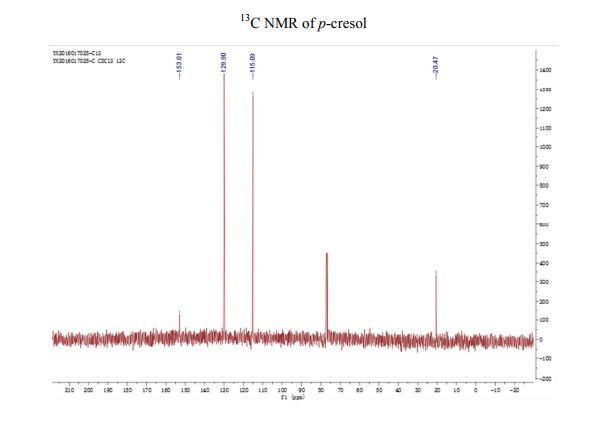
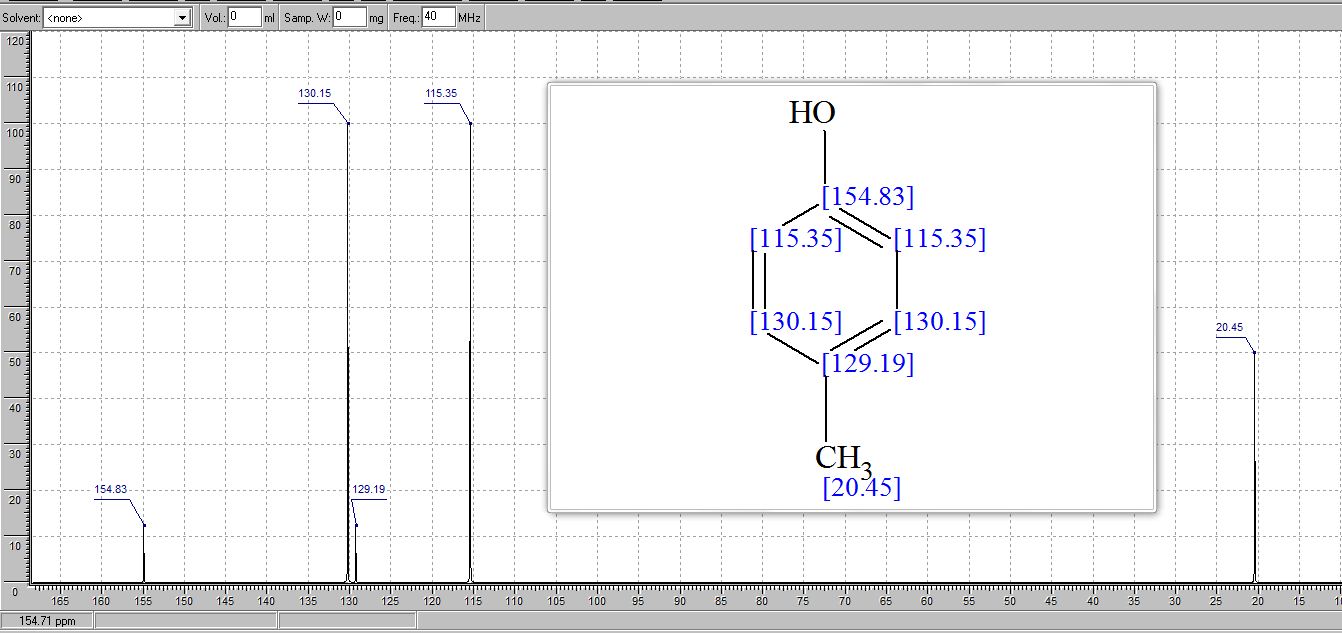
13C NMR (CDCl3) δ/ppm: 153.0, 129.9, 115.1, 20.5.
Literature data:(3b) 1H NMR (300 MHz, CDCl3) δ/ppm: 7.03 (d, J = 8.2 Hz, 2H), 6.73 (dd, J = 8.2, 2.0 Hz, 2H), 4.75 (s, 1H, OH), 2.27 (s, 3H, CH3).
13C NMR (CDCl3) δ/ppm: 153.2, 130.2, 115.2, 20.6.
3(b) Taniguchi, T.; Imoto, M.; Takeda, M.; Nakai, T.; Mihara, M.; Iwai, T.; Ito, T.; Mizuno, T.; Nomoto, A.; Ogawa, A. Heteroat. Chem. 2015, 26, 411– 416 DOI: 10.1002/hc.21275
A Fully Continuous-Flow Process for the Synthesis of p-Cresol: Impurity Analysis and Process Optimization
†National Engineering Research Center for Process Development of Active Pharmaceutical Ingredients, Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, ‡Key Laboratory for Green Pharmaceutical Technologies and Related Equipment of Ministry of Education, College of Pharmaceutical Sciences, Zhejiang University of Technology, Hangzhou 310014, P. R. China
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.7b00250
*Tel.: (+86)57188320899. E-mail: pharmlab@zjut.edu.cn.
NMR PREDICT