TASELISIB
SYNTHESIS........https://newdrugapprovals.org/2018/06/15/rg-7604taselisib/
CLIP
Manufacture of the PI3K β-Sparing Inhibitor Taselisib. Part 2: Development of a Highly Efficient and Regioselective Late-Stage Process
† Department of Small Molecule Process Chemistry, Genentech Inc., 1 DNA Way, South San Francisco, California 94080, United States
‡ Small Molecules Technical Development PTDC-C, F. Hoffmann-La Roche Ltd., Grenzacherstrasse 124, 4070 Basel, Switzerland
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.9b00050
*E-mail: stjean.frederic@gene.com. (F.St.-J.), *E-mail: angelaud.remy@gene.com. (R.A.)
A highly efficient and regioselective manufacturing route for the phosphoinositide 3-kinase β-sparing inhibitor taselisib was developed. Highlights of the synthesis include: (1) magnesium-mediated formation of a challenging cyclic amidine; (2) regioselective imidazole construction via alkylation/condensation with bromopyruvic acid; and (3) triazole formation with 2-isopropyl acetamidrazone to generate the key bromobenzoxazepine core intermediate. Subsequent highly efficient one-pot palladium-catalyzed Miyaura borylation/Suzuki cross-coupling/saponification, followed by a 1,1′-carbonyldiimidazole-mediated coupling with ammonia, led to the pentacyclic taselisib. This new synthetic approach provides a more efficient route to taselisib with a significant decrease in process mass intensity compared to the previous early development routes to the bromobenzoxazepine core. Finally, implementation of a controlled crystallization provided the active pharmaceutical ingredient with the desired polymorphic form.
Taselisib was obtained in 90% yield (16.2 kg) as a white to off-white solid (>99.9 wt %; >99.9 A%) as Form B crystal.
mp (DSC): 257–258 °C.
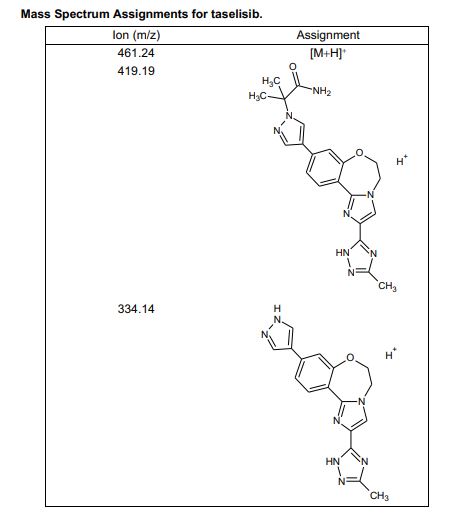
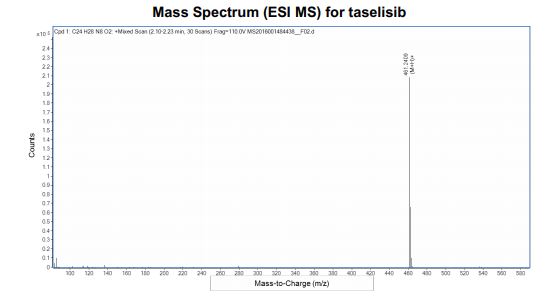
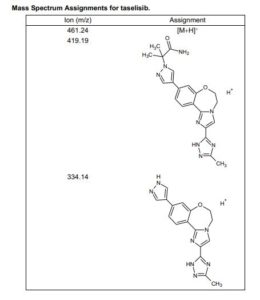
HRMS (ESI-CID) m/z Calcd for [M + H]+ C24H29N8O2: 461.2408; found: 461.2409.
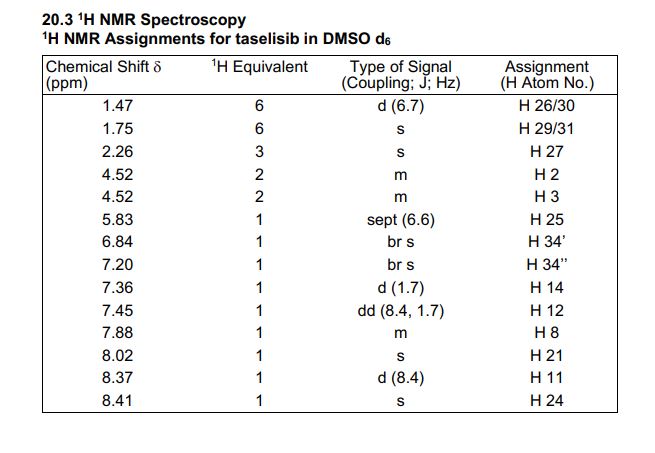
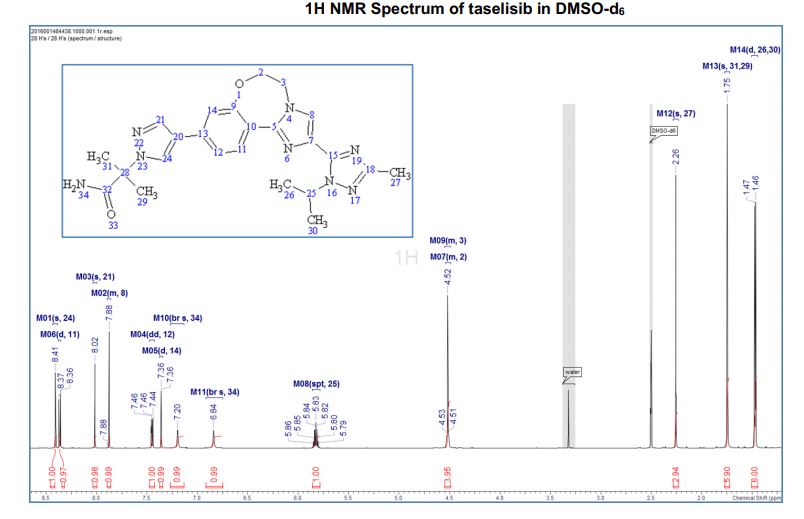
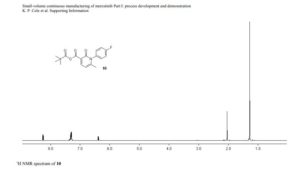
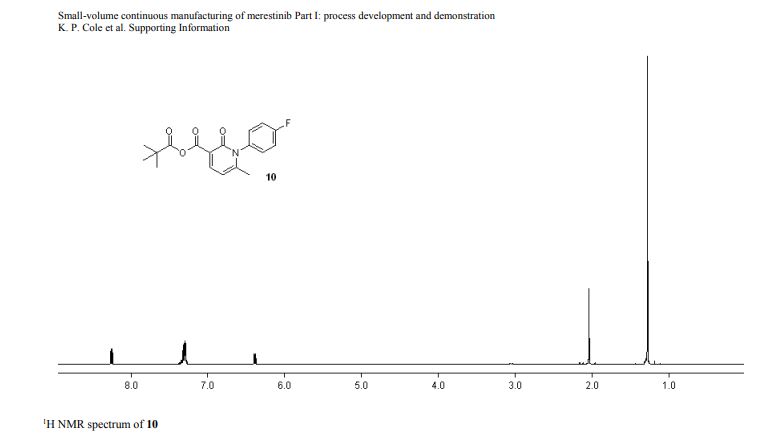
1H NMR (600 MHz, DMSO-d6) δ ppm 8.41 (s, 1H), 8.37 (d, J = 8.4, 1H), 8.02 (s, 1H), 7.88 (m, 1H), 7.45 (dd, J = 8.4, 1.7 Hz, 1H), 7.36 (d, J = 1.7 Hz, 1H), 7.20 (br s, 1H), 6.84 (br s, 1 H), 5.83 (sept, J = 6.6 Hz, 1H), 4.53–4.51 (m, 2H), 4.52 (m, 2H), 2.26 (s, 3H), 1.75 (s, 6H), 1.47 (d, J = 6.7 Hz, 6H).
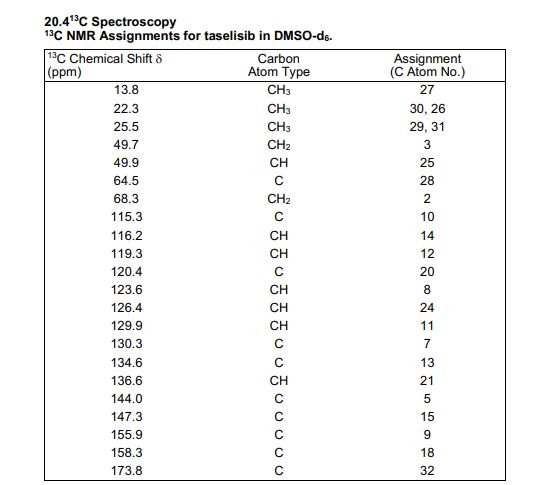
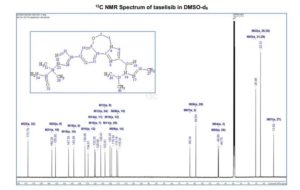
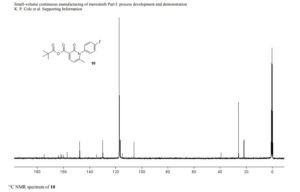
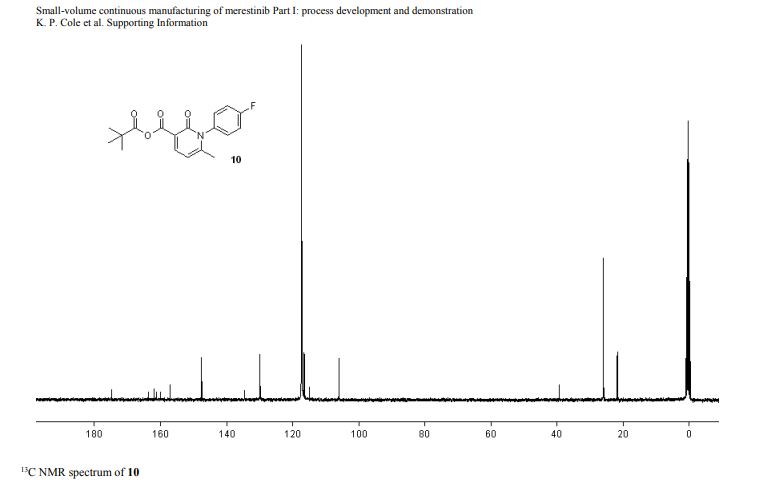
13C NMR (DMSO-d6, 151 MHz) δ = 173.8, 158.3, 155.9, 147.3, 144.0, 136.6, 134.6, 130.3, 129.9, 126.4, 123.6, 120.4, 119.3, 116.2, 115.3, 68.3, 64.5, 49.9, 49.7, 25.5, 25.5, 22.3, 22.3, 13.8.
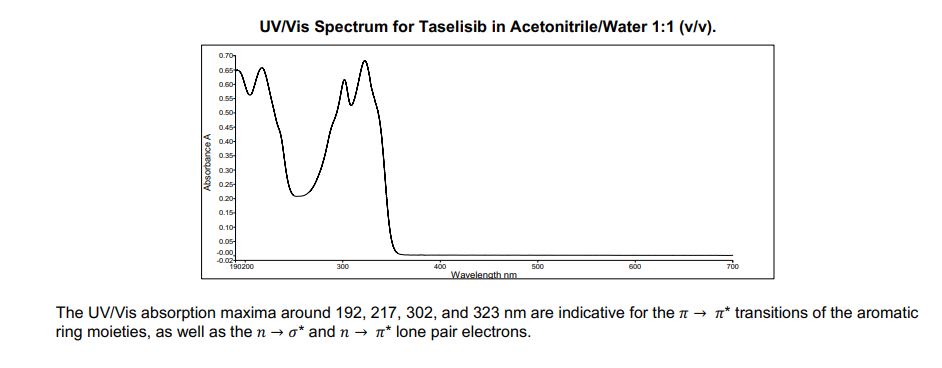
Ultraviolet/Visible Spectroscopy
Apparatus: Perkin Elmer, UV-6190, Lambda 25
Solvent: Acetonitrile/Water 1:1 (v/v)

////////////////
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This is a compilation for educational purposes only. P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
READ
ANTHONY MELVIN CRASTO
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
 amcrasto@gmail.com
amcrasto@gmail.com
CALL +919323115463 INDIA
//////////////













 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..