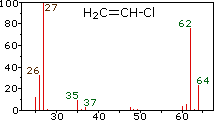
Ospemifene or (Z)-2-[4-(4-chloro- 1 ,2-diphenyl-but- 1 -enyl)phenoxy]ethanol is represented by formula (I):
Ospemifene is an estrogen receptor agonist/antagonist currently investigated e.g. for the treatment of vulvar and vaginal atrophy due to menopause.
Preparation of ospemifene starting from Z-4-(4-hydroxy-l,2-diphenyl-but-l- enyl)phenol has been described in WO 96/07402. Use of McMurry coupling reaction for the manufacture of ospemifene has been described in WO 2008/099059 and WO 2011/089385. These methods suffer from the drawback that large amounts of expensive reagents or solvents, such as titanium tetrachloride, LiAlH4, and 2-Me- THF, are needed.
Actuals...WO2014060639
1H-NMR (400 MHz, CDC13) δ (ppm):
7.37 (2H, t, 7=8Hz, ArH),
7.29 (3Η, t, J=7.2Hz, ArH),
7.20 (2Η, t,7=7.6Hz, ArH),
7.16-7.13 (3Η, m, ArH),
6.80 (2Η, d, J=8.8Hz, ArH),
6.57 (2Η, d, 7=8.8Hz, ArH),
3.94 (2Η, t, y=4.4Hz, ArOCH2CH2OH),
3.87 (2H, m, ArOCH2CH OH),
3.42 (2H, t, J=7.2Hz, C1CH2CH2),
2.92 (2H, t, 7=7.2Hz, C1CH2CH2),
1.95 (1Η, t, 7=6.4Hz, OH).
'
1H NMR PREDICT
ACTUALS 13C NMR.....WO2014060639
13C- NMR (100 MHz, CDC13) δ (ppm):
157.2, 143.2, 142.1 , 141.3, 2 x 135.7, 132.2, 130.0, 129.8, 128.8, 128.7, 127.4, 127.0, 113.9, 69.3, 61.8, 43.3, 39.0.
13C NMR PREDICT
COSY
PATENT
http://www.google.com/patents/WO2014060639A1?cl=en
EXAMPLE 5. Preparation of (Z)-2-[4-(4-chloro-l,2-diphenyl-but-l-enyl)- phenoxy]ethanol (ospemifene) by base hydrolysis of pivaloyl-groiip
; . (Z)-2-(4-(4-Chloro- l ,2-diphenylbut-l-en- l-yl)phenqxy)ethyl pivalate ( 1 g, 2.16 mmol) was dissolved in THF (8 ml) followed by addition of MeOH (1 ml) and water (1 ml). Sodium hydroxide (0.1 g, 2.5 mmol) was added in orie portion and the reaction was stirred at room temperature for 12 h. After completion of the reaction the mixture was partitioned between water (20 ml) and EtOAc (20 ml). Organic phase was washed with water (20 ml) and brine (20 ml); dried (Na2S04), filtered, and concentrated: The residue was crystallized from -PrOH yielding ospernifene (0:29 g, 35 %) as a white solid.
1H-NMR (400 MHz, CDC13) δ (ppm): 7.37 (2H, t, 7=8Hz, ArH), 7.29 (3Η, t, J=7.2Hz, ArH), 7.20 (2Η, t,7=7.6Hz, ArH), 7.16-7.13 (3Η, m, ArH), 6.80 (2Η, d, J=8.8Hz, ArH), 6.57 (2Η, d, 7=8.8Hz, ArH), 3.94 (2Η, t, y=4.4Hz, ArOCH2CH2OH), 3.87 (2H, m, ArOCH2CH OH), 3.42 (2H, t, J=7.2Hz, C1CH2CH2), 2.92 (2H, t, 7=7.2Hz, C1CH2CH2), 1.95 (1Η, t, 7=6.4Hz, OH).
13C- NMR (100 MHz, CDC13) δ (ppm): 157.2, 143.2, 142.1 , 141.3, 2 x 135.7, 132.2, 130.0, 129.8, 128.8, 128.7, 127.4, 127.0, 113.9, 69.3, 61.8, 43.3, 39.0.
EXAMPLE 6. Preparation of (Z)-2-[4-(4-chloro-l,2-diphenyl-but-l-enyl)- phenoxy]ethanol (ospernifene) by reductive cleavage of pivaloyl-grou
(Z)-2-(4-(4-Chloro- 1 ,2-diphenylbut- 1 -en- 1 -yl)phenoxy)ethyl pivalate (3.5 g, 7.56 mmol) was dissolved in toluene (35 ml) and stirred under nitrogen for 5 min at room temperature. Lithium aluminium hydride solution (1 M in THE) (7.56 ml, 7.56 n mbi) was added dropwise to the reaction and the mixture was stirred at room temperature for 30 min. After HPLC indicated completion, the reaction was quenched by addition of saturated NH4Cl-sblution (75 ml). Additional amount of toluene (30 ml) was added and the phases were separated. The organic phase was washed with water (50 ml), brine (50 ml), dried (Na2S04), filtered and concentrated in vacuo. The residue was crystallized from 90 % MeOH yielding ospernifene (1 ,75 g, 61 9c) as a white solid.
1H NMR PREDICT
13C NMR PREDICT
Patent
| Filing date | Publication date | Applicant | Title | |
|---|---|---|---|---|
| WO1996007402A1 | Sep 6, 1995 | Mar 14, 1996 | Michael Degregorio | Triphenylethylenes for the prevention and treatment of osteoporosis |
| WO2008099059A1 | Feb 13, 2008 | Aug 21, 2008 | Hormos Medical Ltd | Method for the preparation of therapeutically valuable triphenylbutene derivatives |
| WO2011089385A1 | Jan 19, 2011 | Jul 28, 2011 | Cambrex Karlskoga Ab | New processes for producing benzophenone derivatives |
H-NMR spectral analysis
CAS NO. 128607-22-7, 2-[4-[(Z)-4-chloro-1,2-diphenylbut-1-enyl]phenoxy]ethanol H-NMR spectral analysis |
C-NMR spectral analysis
CAS NO. 128607-22-7, 2-[4-[(Z)-4-chloro-1,2-diphenylbut-1-enyl]phenoxy]ethanol C-NMR spectral analysis |
http://www.google.com/patents/CN104030896A?cl=en
Ospemifene olefins having molecular structure, W02011 / 89385 by the use of 4-hydroxy-benzophenone (2) and 2-bromoethanol in DMF to give 4-hydroxyethoxy-phenyl benzophenone ( 3), then by (3) and 3-chloro-acetone titanium tetrachloride - zinc catalytic reactions occur McMurry generated directly ospemifene.
[0007]
[0008] The method or W02008 / 099059 uses, hydroxy benzophenone 4_ 3_ chlorobenzene first with acetone to give compound by McMurry reaction of 5-chloro-1,2-diphenyl-1- (4-hydroxyphenyl ) -1_ pentene (4), and then the reaction Williams (5)
And to protect the reaction of ospemifene.
[0009]
The key step [0010] The method is to generate two ketone double bond coupling reaction, called McMurry reaction. This reaction of aldehydes and ketones in the reducing metal (Li, Na, Mg, Zn, LiAlH4, Zn-Cu) and a lower valence state titanium (TiCl3, TiCl4) role two carbonyl groups condensation-deoxy get olefins. Although McMurry reaction step is relatively simple, but the reaction conditions are harsh, self-conjugated cis-trans isomerization is difficult to avoid. Thus prepared ospemifene process, McMurry reaction prone plurality of side reaction products, the quality of which will affect the final product. States pharmacopoeia of a single impurity API has very strict limits, generally require less than 0.1%. In ospemifene of technology and quality research, separation and identification of impurities may have produced, then the structure of impurity, presumably causes and pathways that may arise in the process be avoided. And if the impurity has been generated, depending on the nature of impurities using a good method to remove them, for the preparation of high purity ospemifene has a very big help.
Referring to W02008 / 099059 ospemifene obtained crude product by column chromatography using ethyl acetate: petroleum ether = 1: 3 as eluent to give a white solid. HPLC mobile phase: 0.5% triethylamine (adjusted with phosphoric acid to pH 3.0) - acetonitrile = 60: 40; flow rate: 1.0ml.mirT1; detection wavelength was 277nm. 1HNMR = L 90 (t, 1H, OH), 3.85 (q, 2H, CH2), 3.93 (m, 2H, -CH2), 4.65 (s, 1H,), 6.65 (d, 2H, benzene ring), 6.90 ~ 7.24 (m, 7H, phenyl ring). Elemental analysis = C15H16O3 Calculated C73.75%, H6.60%; Found C73.55%, H6.24%.
[0049] Example 9 ospemifene passivation
[0050] The ospemifene crude 20g added hexane 10ml, 40 ° C with stirring 30min, coolish, standing Ih pour out the liquid, then added hexane 10ml, repeat the above operation. The remaining solvent was evaporated under reduced pressure, the residue was added 160ml of methanol and ethanol were heated and dissolved, cooled to 40 ° C, maintaining the temperature was allowed to stand seeding crystallization. After crystallization, crystallization continued cooling and stirring, and then place the frozen _25 ° C. Filtered to give a white solid 15.2g, yield 76%, HPLC mobile phase: 0.5% triethylamine (adjusted with phosphoric acid to pH 3.0) - acetonitrile = 60: 40; flow rate: 1.0ml.mirT1; detection wavelength was 277nm The content of 99.92%.
10 ospemifene purification Example [0051] Example
[0052] The ospemifene crude 20g cyclohexane 100ml, stirred at room temperature 30min, cooled to (TC, standing Ih pour out the liquid, then cyclohexane 60ml, repeat the operation. Evaporated under reduced pressure The remaining solvent, the residue was added ethanol 200ml dissolved by heating and cooled to 40 ° C, slowly dropping water 20ml, maintaining the temperature was allowed to stand seeding crystallization. After crystallization, crystallization continued cooling and stirring at room temperature 25 ° C place. Filtering to give a white solid 14.8g, yield 74%, HPLC mobile phase:
0.5% triethylamine (adjusted with phosphoric acid to pH 3.0) - acetonitrile = 60: 40; flow rate: 1.0ml.mirT1; detection wavelength was 277nm, the content of 99.89%. [0053] Example 11 ospemifene passivation
[0054] The ospemifene crude 20g heptane was added 50ml, room temperature for 30min, cooled at room temperature, the liquid was decanted standing Ih, then heptane was added 20ml, repeat the above operation. The remaining solvent was distilled off under reduced pressure, the residue was added 160ml of isopropanol was heated to dissolve, cooled to 30 ° C, water was slowly added dropwise 16ml, maintaining the temperature was allowed to stand seeding crystallization. After crystallization, continue stirring and cooled to 0 ° C crystallization, at room temperature. Filtered to give a white solid 15.8g, Yield 79%, HPLC mobile phase:
0.5% triethylamine (adjusted with phosphoric acid to pH 3.0) - acetonitrile = 60: 40; flow rate: 1.0ml.mirT1; detection wavelength was 277nm, the content of 99.85%.
[0055] Example 12 ospemifene passivation
[0056] The above operation ospemifene crude 20g cyclohexane 40ml, room temperature for 30min, cooled at room temperature, the liquid was decanted standing Ih, then cyclohexane 20ml, repeat. The remaining solvent was distilled off under reduced pressure, the residue was added 200ml methanol was dissolved by heating, cooled to 30 ° C, water was slowly added dropwise 20ml, maintaining the temperature was allowed to stand seeding crystallization. After crystal precipitation, continue stirring and cooled to 0 ° C crystallization. Filtered to give a white solid 16.2g, Yield 81%, HPLC mobile phase: 0.5% triethylamine (adjusted with phosphoric acid to pH 3.0) - acetonitrile = 60: 40; flow rate: 1.0ml.mirT1; detection wavelength was 277nm The content of 99.90% ο
[0057] Example 13 ospemifene passivation
[0058] The ospemifene crude 20g of n-hexane was added 200ml, stirred at room temperature 30min, cooled at room temperature, the liquid was decanted standing Ih and then added hexane 20ml, repeat the above operation. The remaining solvent was evaporated under reduced pressure, the residue is added methanol, ethanol and isopropanol mixture was stirred at room temperature 300ml lh, maintaining the temperature was allowed to stand seeding crystallization. Filtered to give a white solid 12.4g, Yield 62%, HPLC mobile phase: 0.5% triethylamine (adjusted with phosphoric acid to pH 3.0) - acetonitrile = 60: 40; flow rate: 1.0ml.mirT1; detection wavelength was 277nm The content of 99.93%.
................
ARTICLE
http://www.yaopha.com/wp-content/uploads/2014/09/%E6%AC%A7%E5%8F%B8%E5%93%8C%E7%B1%B3%E8%8A%AC%E7%9A%84%E4%B8%A4%E6%AD%A5%E6%B3%95%E5%90%88%E6%88%90%E5%B7%A5%E8%89%BA%E7%A0%94%E7%A9%B6.pdf
H-NMR(400 MHZ,CDCl3)δ:2.98(t,
2H,=CH2CH2Cl),3.45(t,2H,=CH2CH2Cl),3.85
(t,2H,-OCH2CH2OH),3.95(t,2H,-OCH2CH2OH),
6.58(d,2H,羟基苯邻位),6.80(d,2H,羟基苯
间位),7.10~7.43(m,10H,苯环)。
13C-NMR(100
MHZ,CDCl3)δ:38.55(C-9),42.80(C-10),61.40
(C-1),68.88(C-2),113.48(C-4),126.95(C-5),
128.20(C-17),128.34(C-13),129.36(C-16),
129.51(C-12),131.73(C-14),135.26(C-8),135.34
(C-7),140.93(C-15),141.65(C-11),142.80(C-6),
156.79(C-3)。合成路线见图 1。
Join me on google plus  Googleplus
Googleplus
 amcrasto@gmail.com
amcrasto@gmail.com
 LIONEL MY SON
LIONEL MY SON
He was only in first standard in school when I was hit by a deadly
one in a million spine stroke called acute transverse mylitis, it made
me 90% paralysed and bound to a wheel chair, Now I keep him as my source
of inspiration and helping millions, thanks to millions of my readers
who keep me going and help me to keep my son happy
सुकून उतना ही देना प्रभू, जितने से
जिंदगी चल जाये।
औकात बस इतनी देना,
कि औरों का भला हो जाये।
जिंदगी चल जाये।
औकात बस इतनी देना,
कि औरों का भला हो जाये।
////////////