Isotopes: Br and Cl
Isotopes: Br and Cl
There
are other cases in which knowledge of isotopes can be crucial. For
example, bromoethane should display a peak for the molecular ion at m/z =
109. However, the farthest large peak to the right in its mass spectrum
is at 110. There is another large peak at 108.
Figure MS4. Mass spectrum of bromoethane. Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 22 August 2008)
- In the periodic table, the atomic mass of bromine is listed as 80, but that is just an average.
- Bromine is really about 50% 79Br and 50% 81Br.
- As a result, two molecular ion peaks of equal intensity two units apart, M+ and M+2, are observed.
- This pattern of molecular ions is a good indication that there is a bromine present in the molecule.
Similarly,
chlorobenzene should display M+ at m/z =112, provided you take into
account that most chlorine atoms have an atomic weight of 35 amu. The
periodic table lists an atomic weight for chlorine of 35.453 amu,
though. That's because about 25% of chlorine atoms are actually 37Cl. The mass spectrum of chlorobenzene actually shows an additional molecular ion at 114 amu.
Figure MS5. Mass spectrum of chlorobenzene.
Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 22 August 2008)
- Chlorinated compounds show an M+2 peak that is 1/3 as large as the M+ peak.
Note
also that halogens are easily lost during mass spectrometry. If you
subtract the mass of the halogen from the molecular ion mass, you will
often find a peak that corresponds to the remainder of the structure.
Problem MS4.
Draw one possible structure for the compound in each of the following mass spectra.
Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 25 August 2008)
Problem MS5.
In the following mass spectrum, more than one halogen atom is present.
- What is a possible structure of the compound?
- Show why this pattern of molecular ions is observed.
Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 25 August 2008)
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
 LIONEL MY SON
LIONEL MY SON

Isotopes
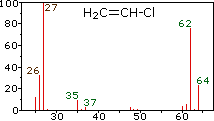
Since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. This is manifested most dramatically for compounds containing bromine and chlorine, as illustrated by the following examples. Since molecules of bromine have only two atoms, the spectrum on the left will come as a surprise if a single atomic mass of 80 amu is assumed for Br. The five peaks in this spectrum demonstrate clearly that natural bromine consists of a nearly 50:50 mixture of isotopes having atomic masses of 79 and 81 amu respectively. Thus, the bromine molecule may be composed of two 79Br atoms (mass 158 amu), two 81Br atoms (mass 162 amu) or the more probable combination of 79Br-81Br (mass 160 amu). Fragmentation of Br2 to a bromine cation then gives rise to equal sized ion peaks at 79 and 81 amu.
Since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. This is manifested most dramatically for compounds containing bromine and chlorine, as illustrated by the following examples. Since molecules of bromine have only two atoms, the spectrum on the left will come as a surprise if a single atomic mass of 80 amu is assumed for Br. The five peaks in this spectrum demonstrate clearly that natural bromine consists of a nearly 50:50 mixture of isotopes having atomic masses of 79 and 81 amu respectively. Thus, the bromine molecule may be composed of two 79Br atoms (mass 158 amu), two 81Br atoms (mass 162 amu) or the more probable combination of 79Br-81Br (mass 160 amu). Fragmentation of Br2 to a bromine cation then gives rise to equal sized ion peaks at 79 and 81 amu.

|  |  | ||
| bromine | vinyl chloride | methylene chloride |
|---|
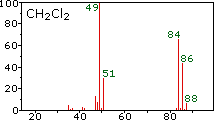
The center and right hand spectra show that chlorine is also composed
of two isotopes, the more abundant having a mass of 35 amu, and the
minor isotope a mass 37 amu. The precise isotopic composition of
chlorine and bromine is:
Chlorine: 75.77% 35Cl and 24.23% 37Cl
Bromine: 50.50% 79Br and 49.50% 81Br
Chlorine: 75.77% 35Cl and 24.23% 37Cl
Bromine: 50.50% 79Br and 49.50% 81Br
The presence of chlorine or bromine in a molecule or ion is easily
detected by noticing the intensity ratios of ions differing by 2 amu. In
the case of methylene chloride, the molecular ion consists of three
peaks at m/z=84, 86 & 88 amu, and their diminishing intensities may
be calculated from the natural abundances given above. Loss of a
chlorine atom gives two isotopic fragment ions at m/z=49 & 51amu,
clearly incorporating a single chlorine atom.
Fluorine and iodine, by contrast, are monoisotopic, having masses of 19
amu and 127 amu respectively. It should be noted that the presence of
halogen atoms in a molecule or fragment ion does not change the odd-even
mass rules given above.
| email me .......amcrasto@gmail.com |
|---|
Two other common elements having useful isotope signatures are carbon, 13C is 1.1% natural abundance, and sulfur, 33S and 34S
are 0.76% and 4.22% natural abundance respectively. For example, the
small m/z=99 amu peak in the spectrum of 4-methyl-3-pentene-2-one
(above) is due to the presence of a single 13C atom in the molecular ion. Although less important in this respect, 15N and 18O also make small contributions to higher mass satellites of molecular ions incorporating these elements.
The calculator on the right may be used to calculate the isotope
contributions to ion abundances 1 and 2 amu greater than the molecular
ion (M). Simply enter an appropriate subscript number to the right of
each symbol, leaving those elements not present blank, and press the "Calculate" button. The numbers displayed in the M+1 and M+2 boxes are relative to M being set at 100%.
Join me on google plus  Googleplus
Googleplus
 amcrasto@gmail.com
amcrasto@gmail.com
 LIONEL MY SON
LIONEL MY SON
He was only in first standard in school when I was hit by a deadly
one in a million spine stroke called acute transverse mylitis, it made
me 90% paralysed and bound to a wheel chair, Now I keep him as my source
of inspiration and helping millions, thanks to millions of my readers
who keep me going and help me to keep my son happy
सुकून उतना ही देना प्रभू, जितने से
जिंदगी चल जाये।
औकात बस इतनी देना,
कि औरों का भला हो जाये।
जिंदगी चल जाये।
औकात बस इतनी देना,
कि औरों का भला हो जाये।
///////
No comments:
Post a Comment