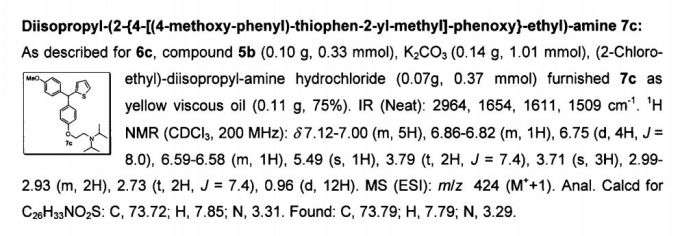
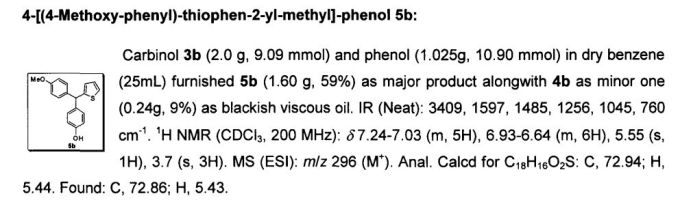
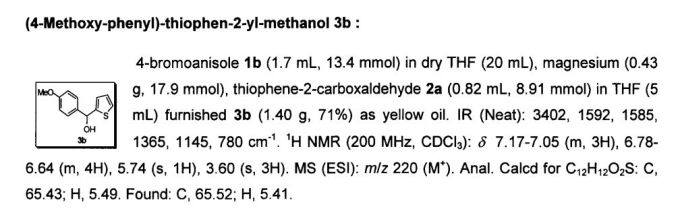
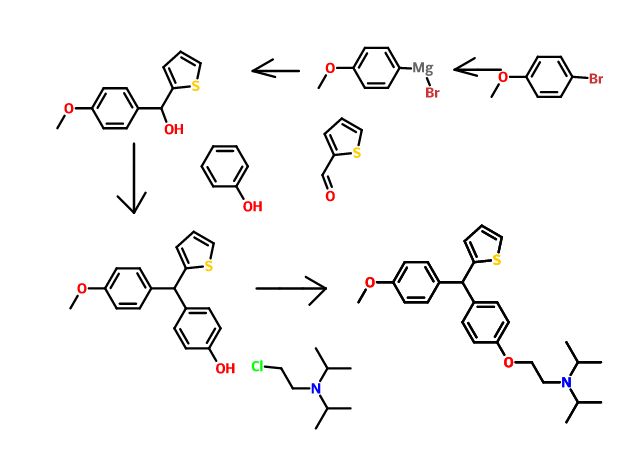
Development of a Practical Process for the Synthesis of PDE4 Inhibitors
Chemical Development US, Boehringer Ingelheim Pharmaceuticals, Inc., 900 Ridgebury Road, P.O. Box 368, Ridgefield, Connecticut 06877-0368, United States
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.6b00104
Publication Date (Web): April 28, 2016
Copyright © 2016 American Chemical Society
/////////
Vis island krka park, croatia
Vis
Island in the Adriatic Sea
Vis
is a small Croatian island in the Adriatic Sea. The farthest inhabited
island off the Croatian mainland, Vis had a population of 3,460 in 2011
and has an area of 90.26 square kilometres. Wikipedia
Vis island & krka park, croatia - swimming in secret bays and waterfalls
Fifty kuna (eight bucks) will get you on a Jadrolinija ferry to Hvar, Brač, Korčula, or Šolta -- all lush paradises with perfect golden beaches, fresh seafood, colorful port towns and unspoiled nature galore.
Pulling away from Split...
... and into Vis.
Vis is the furthest of the Dalmatian islands from the coast, and its isolation, coupled with its history (until the late 1980s) as a Yugoslav military base, means the island has avoided over-development (or, really, much development at all) and preserved its abundant natural beauty.
Arriving on the ferry, I wanted to take off on foot to find Stiniva Beach, had nothing but a very rough idea of what direction to go in, and was completely unaware that we'd be walking along the side of the only road, a tiny winding wisp of pavement which locals took at six hundred kilometeres per hour in their rickety old Fiats.
Sara put her foot down and demanded we figure out how far away Stiniva was from the port (quite far) and whether we could get there on foot (no). Off to hail a taxi.
Me, to taxi driver: "We'd like to go to Stiniva Beach."
Taxi driver: *looks down at my breasts* *looks up at my face* "Yes."
Our charming driver winged around the sharp turns, careening over terrifying cliffs and through idyllic grape fields, while blasting at top volume, on repeat, LMFAO's 'Champagne Showers'. It was a jarring experience.
Arriving at the little sign for Stiniva Beach, we had a 20 minute descent about 85 degrees straight down a cliff face.
With every step, the beach comes a little more into view -- we're headed to that yellow roof near the bottom.
All worth it, the second your feet touch sand -- er, rocks.
We ripped off our clothes and jumped in.
And did a little exploring. There were folks snorkeling, solo climbing, and cliff jumping, and we did a bit of everything (though our "solo climbing" efforts were much less majestic than the actual rock climber, and consisted mostly of stepping extremely carefully around urchins).
Sara bravely takes a leap!
There's a little shack on the beach. When we walked over to ask if they had food, the merry surfer dues running the joint said, "Sure, we got a cook! What do you want?"
We shelled out a couple bucks for cold beers to go.
Before we knew it, it was time to make the ascent back up the rock face to meet our favorite cabbie.
Goodbyes are so hard.
We were plenty early for our ferry, so wandered around the town at the port a bit, soaking every last bit in.
Last glance of Vis as the ferry pulls out from the port.
Pulling into Split at sunset.
If you can hack it, make it to Vis Island, and to Stiniva Beach. It's the most spectacular place I've ever swam in, and in the past year, I've racked up a startling amount of good secret swimming holes. Don't be deterred by the steep, perilous climb. Get there early or stay late to avoid rush hour of tourist boats cramming into the bay. And bring a couple bucks to buy a cold beer.
Back in Split, we got a huge take out pizza a piece and a cold bottle of bubbly and ate on the port, laughing for hours, having funny conversations with strangers who passed by. It was a warm night and everyone was happy and in a good mood. This is my kind of nightlife.
Another day, we set out to check off a big item on Sara's bucket list: swimming at the base of a waterfall.
We took a bus into Split, then a bus to Krka National Park, and then a ferry to get to the waterfalls.
We chose Krka over the more popular but remarkably beautiful Plitvice just to cut out four hours on a bus. It was a beautiful day, perfect for a cool swim.
The park is built around the 73km Krka River, which carved a path through limestone hills forming a 200m deep canyon. Calcium carbonate buildup causes billions of plants growing on top of one another to form a barrier that produces the spectacular 800m waterfalls.
Sara, dream list to-do crossed off, blissed out.
They're not hanging out in a perfectly straight line for fun (we're not in Switzerland. ZING) -- the falls are roped off, and risk takers are confronted with a speed boat manned by vicious tanned teenage lifeguards who guide you back behind the ropes.
Sara was in heaven, and me.... this is not my scene. No words can express how massively crowded this place was -- but maybe the photo below can. People had spread out blankets on the fields next to the river, and people were essentially shoulder to shoulder, blanket edges overlapping.
I like my space and my solitude when hanging out in nature, and Krka at 2pm on a Monday just before August was.... the opposite of that.
Sara and I had been warned of the crowds (Lonely Planet Croatia: "insanely busy"), and vowed to arrive early. We left our apartment in the boondocks at 6am, and, after a bus into the city, a bus to Skradin, and then humongous lines for park entrance tickets and then the same humongous line to catch the ferry to the waterfalls, we got there at 1pm. Pro tip: wake up well before sunrise, splurge for a taxi, get there early. It'd be worth it.
Unless you're a person like Sara, of course, an endless well of zen positivity.
There is a path that cuts around and through the park (about an hour long), which we hiked for a respite from the crowds.
Selfie with the 800m Skradinski Buk -- the waterfalls -- behind us.
Next week, one last post from Croatia: downtown Split.
All photos taken by either Sara or me -- we can't remember whose is whose anymore!
////////






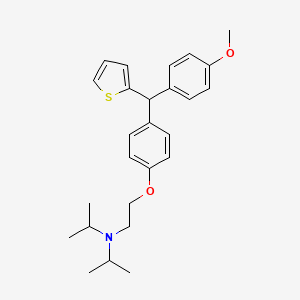



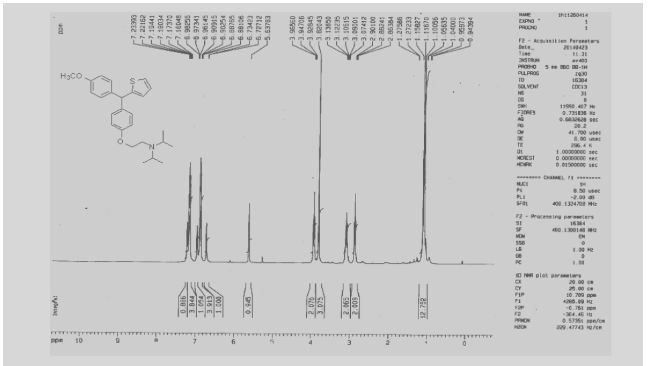
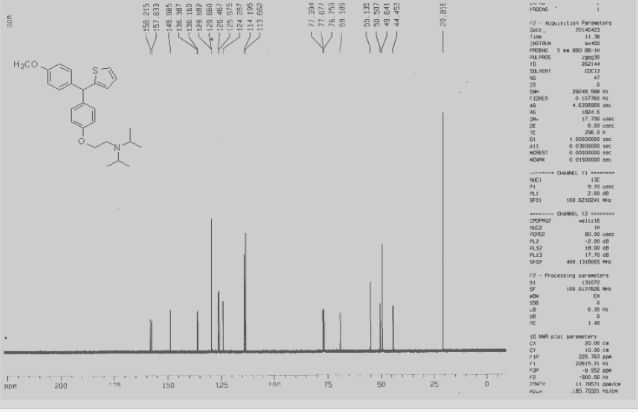



 .
.












