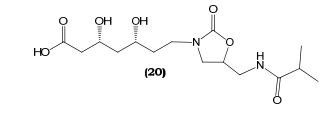
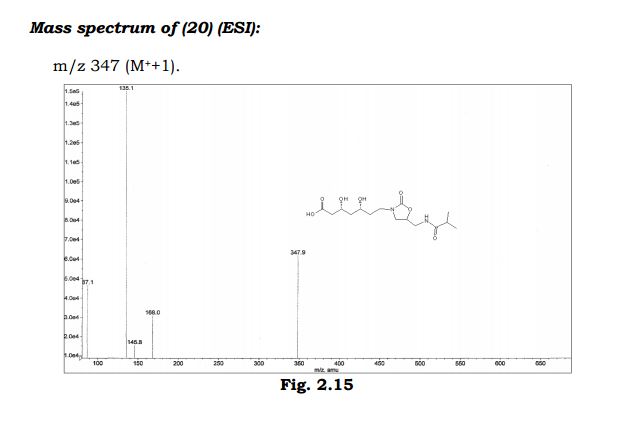
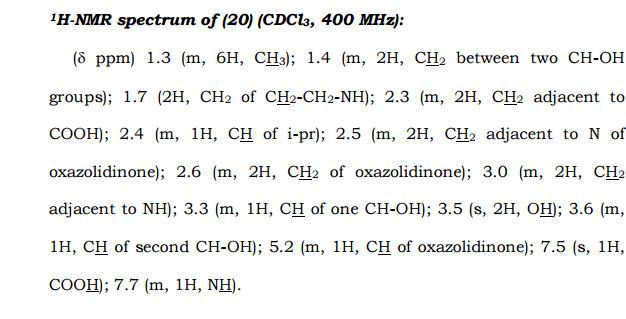
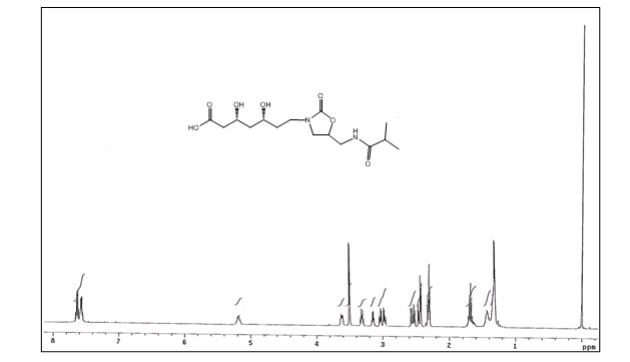
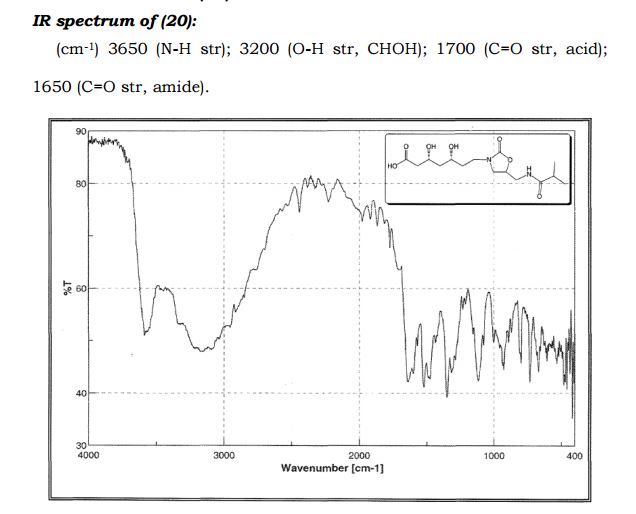
(3R,5R)-3,5-dihydroxy-7-(5-(isobutyramidomethyl)-2- oxooxazolidin-3-yl)heptanoic acid
Organic Chemists from Industry and academics to Interact on Spectroscopy Techniques for Organic Compounds ie NMR, MASS, IR, UV Etc. Starters, Learners, advanced, all alike, contains content which is basic or advanced, by Dr Anthony Melvin Crasto, Worlddrugtracker, email me ........... amcrasto@gmail.com, call +91 9323115463 India skype amcrasto64
................DR ANTHONY MELVIN CRASTO Ph.D ( ICT, Mumbai) , INDIA 25Yrs Exp. in the feld of Organic Chemistry,Working for GLENMARK GENERICS at Navi Mumbai, INDIA. Serving chemists around the world. Helping them with websites on Chemistry.Million hits on google, world acclamation from industry, academia, drug authorities for websites, blogs and educational contribution
Pages
- Home
- ABOUT ME
- DIMENSIONS IN NMR SPECTROSCOPY
- 13 C NMR
- 1H NMR
- CHEMDOODLE/INTERACTIVE SPECT PREDICT
- Animations
- HELP ME
- Multinuclear NMR Spectroscopy
- Examples of 13C NMR
- Books on NMR spectroscopy
- UV-Visible Spectroscopy
- IR SPECTRA EXAMPLES
- Journals
- Organic spectroscopy site
- Spectroscopy sites
- IR SPECTROSCOPY
- Books-2
- Recommended Web Sites for Spectra and Spectrum-rel...
- DISCLAIMER
- Mössbauer spectroscopy
- FINDING CHEMICAL SPECTRA
- Mass Spectrometry
- NMR Overview
- Characterisation of Organic Compounds
- SDBS Spectral Database System for Organic Compounds
- CHEMICAL SHIFT
- MASS SPECTROSCOPY
- Books-1
- MASSBANK PORTAL
- 11B NMR
Tuesday, 2 August 2016
Monday, 1 August 2016
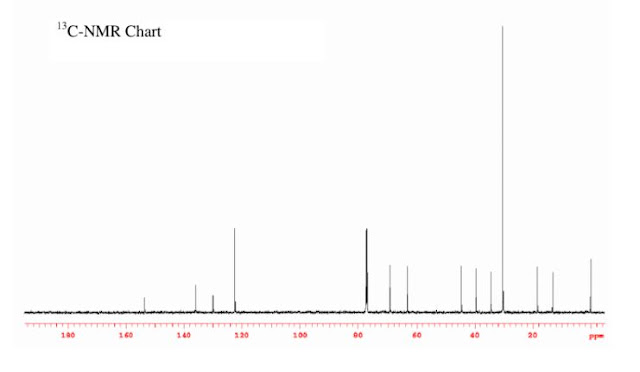
1-chloro-4-(3,5-dichloropent-1-ynyl)benzene

1-chloro-4-(3,5-dichloropent-1-ynyl)benzene (3ci) :
Following general procedure, The product was purified by flash column chromatography on silica gel (petroleum ether) and 1c : 2i = 1:53, obtained in 61 % yield as a pale yellow oil (29.9 mg).
1H NMR (600 MHz, CDCl3) δ 7.37 (t, J = 10.3 Hz, 2H), 7.31 (d, J = 8.2 Hz, 2H), 5.00 (t, J = 6.8 Hz, 1H), 3.78 (ddd, J = 16.9, 10.1, 4.7 Hz, 2H), 2.47 (q, J = 6.5 Hz, 2H).
13C NMR (151 MHz, CDCl3) δ 135.1, 133.0, 128.7, 120.2, 86.9, 85.6, 45.9, 41.4, 40.8.
HRMS calcd for C11H9Cl3 [M + H]+ 245.9770; found: 245.9772.
Directed alkynylation of unactivated C(sp3)-H bonds with ethynylbenziodoxolones mediated by DTBP
Green Chem., 2016, 18,4185-4188
DOI: 10.1039/C6GC01336H, Communication
Zhi-Fei Cheng, Yi-Si Feng, Chun Rong, Tao Xu, Peng-Fei Wang, Jun Xu, Jian-Jun Dai, Hua-Jian Xu
A general and efficient alkynylation of unactivated C(sp3)-H bonds under metal-free conditions was developed herein.
A general and efficient alkynylation of unactivated C(sp3)-H bonds under metal-free conditions was developed herein.
Directed alkynylation of unactivated C(sp3)–H bonds with ethynylbenziodoxolones mediated by DTBP
Zhi-Fei Cheng,a Yi-Si Feng,*abc Chun Rong,a Tao Xu,a Peng-Fei Wang,a Jun Xu,a Jian-Jun Daia and Hua-Jian Xu*abc
*Corresponding authors
aSchool of Chemistry and Chemical Engineering, School of Biological and Medical Engineering, Hefei University of Technology, Hefei 230009, P. R. China
bAnhui Key Laboratory of Controllable Chemical Reaction and Material Chemical Engineering, Hefei 230009, P. R. China
E-mail: hjxu@hfut.edu.cn
Fax: (+86)-551-62904405
E-mail: hjxu@hfut.edu.cn
Fax: (+86)-551-62904405
cAnhui Provincial Laboratory of Heterocyclic Chemistry, Maanshan 243110, China
Green Chem., 2016,18, 4185-4188
DOI: 10.1039/C6GC01336H, http://pubs.rsc.org/en/Content/ArticleLanding/2016/GC/C6GC01336H?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract
A general and efficient method for the direct alkynylation of unactivated C(sp3)–H bonds under metal-free conditions is described. The reaction performs smoothly under mild conditions and shows excellent functional-group tolerance. Initial mechanistic investigation indicates that the reaction may involve a radical pathway.
//////////Directed alkynylation, unactivated C(sp3)-H bonds, ethynylbenziodoxolones, DTBP
Directed alkynylation of unactivated C(sp3)-H bonds with ethynylbenziodoxolones mediated by DTBP
Directed alkynylation of unactivated C(sp3)-H bonds with ethynylbenziodoxolones mediated by DTBP
Green Chem., 2016, 18,4185-4188
DOI: 10.1039/C6GC01336H, Communication
Zhi-Fei Cheng, Yi-Si Feng, Chun Rong, Tao Xu, Peng-Fei Wang, Jun Xu, Jian-Jun Dai, Hua-Jian Xu
A general and efficient alkynylation of unactivated C(sp3)-H bonds under metal-free conditions was developed herein.
A general and efficient alkynylation of unactivated C(sp3)-H bonds under metal-free conditions was developed herein.
Directed alkynylation of unactivated C(sp3)–H bonds with ethynylbenziodoxolones mediated by DTBP
Zhi-Fei Cheng,a Yi-Si Feng,*abc Chun Rong,a Tao Xu,a Peng-Fei Wang,a Jun Xu,a Jian-Jun Daia and Hua-Jian Xu*abc
*Corresponding authors
aSchool of Chemistry and Chemical Engineering, School of Biological and Medical Engineering, Hefei University of Technology, Hefei 230009, P. R. China
bAnhui Key Laboratory of Controllable Chemical Reaction and Material Chemical Engineering, Hefei 230009, P. R. China
E-mail: hjxu@hfut.edu.cn
Fax: (+86)-551-62904405
E-mail: hjxu@hfut.edu.cn
Fax: (+86)-551-62904405
cAnhui Provincial Laboratory of Heterocyclic Chemistry, Maanshan 243110, China
Green Chem., 2016,18, 4185-4188
DOI: 10.1039/C6GC01336H, http://pubs.rsc.org/en/Content/ArticleLanding/2016/GC/C6GC01336H?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract
A general and efficient method for the direct alkynylation of unactivated C(sp3)–H bonds under metal-free conditions is described. The reaction performs smoothly under mild conditions and shows excellent functional-group tolerance. Initial mechanistic investigation indicates that the reaction may involve a radical pathway.
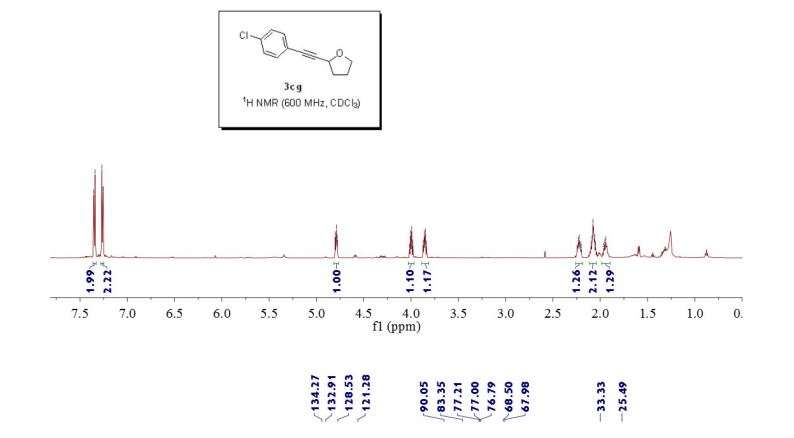
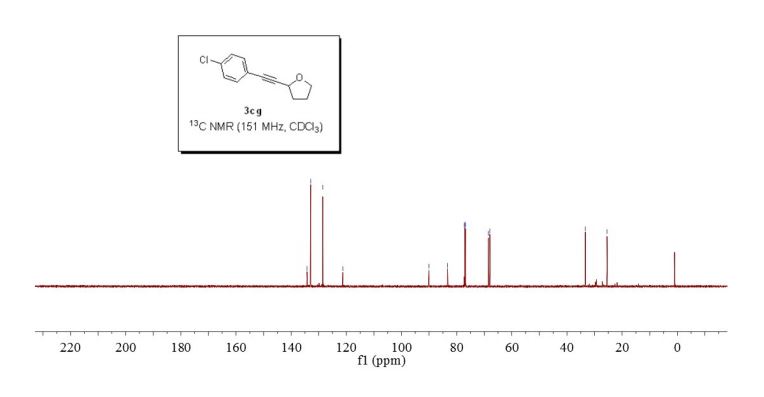
2-((4-chlorophenyl)ethynyl)tetrahydrofuran (3cg) ref 1 : Following general procedure, The product was purified by flash column chromatography on silica gel (petroleum ether) and 1c : 2g = 1:69, obtained in 70 % yield as a pale yellow oil (28.8 mg).
1H NMR (600 MHz, CDCl3) δ 7.35 (d, J = 8.4 Hz, 2H), 7.28 – 7.25 (m, 2H), 4.82 – 4.77 (m, 1H), 4.00 (dd, J = 14.6, 7.1 Hz, 1H), 3.85 (dd, J = 13.6, 7.8 Hz, 1H), 2.26 – 2.19 (m, 1H), 2.11 – 2.04 (m, 2H), 1.95 (dd, J = 13.3, 5.8 Hz, 1H).
13C NMR (151 MHz, CDCl3) δ 134.2, 132.9, 128.5, 121.2, 90.0, 83.3, 68.5, 67.9, 33.3, 25.4.
//////////Directed alkynylation, unactivated C(sp3)-H bonds, ethynylbenziodoxolones, DTBP
THREO ERYTHRO ISOMERS EXAMPLES
(5 R*,1′R*)-5-[3,5-Di-tert-butyl-4-hydroxyphenyl)hydroxymethyl]-2-ethyl-1,2-isothiazolidine-1,1-dioxide 12a (threo) and (5S*,1′R*)-5-[3,5-Di-tert-butyl-4-hydroxyphenyl)hydroxymethyl]-2-ethyl-1,2-isothiazolidine-1,1-dioxide 12b (erythro)
Compound 12a: mp 160−162 °C. 1H NMR (500 MHz, (CDCl3 δ) 1.25 (t, J = 7.3 Hz, 3H), 1.43 (s, 18H), 1.84 (m, 1H), 1.94 (m, 1H), 3.03 (ddd,J = 9.1, 8.2, 7.2 Hz, 1H), 3.08 (dq, J = 13.4, 7.2 Hz, 1H), 3.18 (ddd, J = 9.1, 8.0, 3.5 Hz, 1H), 3.23 (dq, J = 13.4, 7.2 Hz, 1H), 3.53 (m, 1H), 4.84 (d, J = 9.7 Hz 1H), 5.27 (s, 1H), 7.16 (s, 2H). 13C NMR (150 MHz, (CDCl3 δ) 13.18, 23.09, 30.24, 34.41, 39.42, 43.92, 63.67, 74.64, 123.58, 129.99, 136.38, 154.22. Elemental analysis: Calcd for C20H33O4NS: C; 62.63, H; 8.67, N; 3.65, S; 8.36, Found: C; 62.58, H; 8.62, N; 3.66, S; 8.32. Compound 12b: mp 78−96 °C. 1H NMR (500 MHz, (CDCl3 δ) 1.25 (t, J = 7.3 Hz, 3H), 1.44 (s, 18H), 2.08 (m, 1H), 2.60 (dq, J = 13.0, 8.5 Hz, 1H), 3.07 (m, 1H), 3.08 (m, 1H), 3.21 (dq, J = 13.3, 7.2 Hz, 1H), 3.28 (m, 1H), 3.31 (d, J = 2.6 Hz, 1H), 3.38 (td, J = 8.5, 2.1 Hz 1H), 5.21 (s, 1H), 5.40 (brs, 1H), 7.15 (s, 2H). 13C NMR (150 MHz, (CDCl3 δ) 13.18, 18.30, 30.28, 34.43, 39.46, 44.56, 63.21, 69.19, 122.41, 129.94, 136.14, 153.54. Elemental analysis: Calcd for C20H33O4NS: C; 62.63, H; 8.67, N; 3.65, S; 8.36, Found. C; 62.19, H; 8.63, N; 3.62, S; 8.10.
PAPER
Development of One-Pot Synthesis of New Antiarthritic Drug Candidate S-2474 with High E-Selectivity
Katsuo Oda†, Takemasa Hida*†, Teruo Sakata‡, Masahiko Nagai‡, Yoshihide Sugata‡, Toshiaki Masui† and Hideo Nogusa†
Chemical Development Department, CMC Development Laboratories, Shionogi & Co., Ltd., 1-3, Kuise Terajima 2-chome, Amagasaki, Hyogo 660-0813, Japan, and Shionogi Research Laboratories, Shionogi & Co., Ltd., 12-4, Sagisu 5-chome, Fukushima-ku, Osaka 553-0002, Japan
Org. Process Res. Dev., 2008, 12 (3), pp 442–446
DOI: 10.1021/op800008w
* To whom correspondence should be addressed. Telephone: +81-6-6401-8198 . Fax: +81-6-6401-1371. E-mail:takemasa.hida@shionogi.co.jp., †
Chemical Development Department, CMC Development Laboratories.
, ‡Shionogi Research Laboratories.
A one-pot synthesis of S-2474 was developed to overcome the problems of a large number of steps, low stereoselectivity, low yield, a large amount of waste, and severe reaction conditions. Aldol-type condensation of 3,5-di-tert-butyl-4-hydroxybenzaldehyde and N-ethyl-γ-sultam was carried out with LDA and then quenched with water. Dehydration proceeded under basic conditions, providing S-2474 directly as a single isomer on the benzylidene double bond. The reaction mechanism appears to involve a quinone methide intermediate. Environmental assessment of the development of this compound is also discussed in this paper.
?///////New, Antiarthritic , Drug Candidate, S-2474, Shionogi Research Laboratories, cyclooxygenase-2, (COX-2), 5-lipoxygenase , (5-LO),
Sunday, 31 July 2016
5-Fluoro-2-(4-(methylsulfonyl)-phenyl)pyridine
5-Fluoro-2-(4-(methylsulfonyl)-phenyl)pyridine
1H NMR (400 MHz, DMSO-d6) δ 8.73 (d, J = 2.8 Hz, 1H), 8.31 (bd, J = 8.8 Hz, 2H), 8.20 (dd, J = 8.8, 4.3 Hz, 1H), 8.04 (bd, J = 8.5 Hz, 2H), 7.91 (td, J = 8.8, 3.0 Hz, 1H), 3.27 (s, 3H).
13C (100.6 MHz, DMSO-d6) 158.3, 151.3, 142.7, 141.3, 138.4, 128.0, 127.8, 125.0, 124.8, 123.3, 123.2, 44.0.
HRMS calcd for C12H11FNO2S (M + H)+ 252.0489, found, 252.0485.
Paper
Development of Large-Scale Routes to Potent GPR119 Receptor Agonists
Richard T. Matsuoka*†, Eric E. Boros#, Andrew D. Brown†, Kae M. Bullock†, Will L. Canoy‡, Andrew J. Carpenter#, Jeremy D. Cobb†,Shannon E. Condon†, Nicole M. Deschamps†, Vassil I. Elitzin†, Greg Erickson†,Jing M. Fang#, David H. Igo§, Biren K. Joshi‡, Istvan W. Kaldor#, Mark B. Mitchell†, Gregory E. Peckham#, Daniel W. Reynolds‡, Matthew C. Salmon†, Matthew J. Sharp†, Elie A. Tabet#, Jennifer F. Toczko†, Lianming Michael Wu‡, and Xiao-ming M. Zhou†
†API Chemistry Department, ‡Analytical Science & Development Department, #Medicinal Chemistry Department, and§Particle Sciences and Engineering Department, GlaxoSmithKline, 709 Swedeland Road, King of Prussia, Pennsylvania 19406, United States
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.6b00157, http://pubs.acs.org/doi/abs/10.1021/acs.oprd.6b00157
Publication Date (Web): July 13, 2016
Copyright © 2016 American Chemical Society
*E-mail: richard.t.matsuoka@gsk.com.
Abstract
Practical and scalable syntheses were developed that were used to prepare multikilogram batches of GSK1292263A (1) and GSK2041706A (15), two potent G protein-coupled receptor 119 (GPR119) agonists. Both syntheses employed relatively cheap and readily available starting materials, and both took advantage of an SNAr synthetic strategy.
//////////
Subscribe to:
Comments (Atom)