Organic Chemists from Industry and academics to Interact on Spectroscopy Techniques for Organic Compounds ie NMR, MASS, IR, UV Etc. Starters, Learners, advanced, all alike, contains content which is basic or advanced, by Dr Anthony Melvin Crasto, Worlddrugtracker, email me ........... amcrasto@gmail.com, call +91 9323115463 India skype amcrasto64
................DR ANTHONY MELVIN CRASTO Ph.D ( ICT, Mumbai) , INDIA 25Yrs Exp. in the feld of Organic Chemistry,Working for GLENMARK GENERICS at Navi Mumbai, INDIA. Serving chemists around the world. Helping them with websites on Chemistry.Million hits on google, world acclamation from industry, academia, drug authorities for websites, blogs and educational contribution
Pages
- Home
- ABOUT ME
- DIMENSIONS IN NMR SPECTROSCOPY
- 13 C NMR
- 1H NMR
- CHEMDOODLE/INTERACTIVE SPECT PREDICT
- Animations
- HELP ME
- Multinuclear NMR Spectroscopy
- Examples of 13C NMR
- Books on NMR spectroscopy
- UV-Visible Spectroscopy
- IR SPECTRA EXAMPLES
- Journals
- Organic spectroscopy site
- Spectroscopy sites
- IR SPECTROSCOPY
- Books-2
- Recommended Web Sites for Spectra and Spectrum-rel...
- DISCLAIMER
- Mössbauer spectroscopy
- FINDING CHEMICAL SPECTRA
- Mass Spectrometry
- NMR Overview
- Characterisation of Organic Compounds
- SDBS Spectral Database System for Organic Compounds
- CHEMICAL SHIFT
- MASS SPECTROSCOPY
- Books-1
- MASSBANK PORTAL
- 11B NMR
Sunday, 29 October 2017
Saturday, 28 October 2017
(S)-2-(4-Chlorobenzoyl)-1,2,3,4-tetrahydrobenzo[e]pyrazino[1,2-a][1,4]diazepine-6,12(11H,12aH)-dione—Synthesis and Crystallographic Studies
Synthesis of the (S)-2-(4-Chlorobenzoyl)-1,2,3,4-tetrahydrobenzo[e]pyrazino[1,2-a][1,4]diazepine-6,12(11H,12aH)-dione (4)
(S)-piperazine-2-carboxylic acid dihydrochloride (5, 700 mg, 3.45 mmol, 1 equiv.) was dispersed in 50 mL of 1:1 water:dioxane mixture and treated with sodium hydroxide (276 mg, 6.89 mmol, 2 equiv.). After dissolution of the starting material, 4-chlorobenzoyl chloride (6, 0.49 mL, 3.79 mmol, 1.1 equiv.) was added and reaction mixture was stirred in room temperature for 18 h. The next day, the disappearance of starting material and formation of (S)-4-(4-chlorobenzoyl)piperazine-2-carboxylic acid (7) was confirmed by LRMS-ESI spectra. Then, isatoic anhydride (8, 1.69 g, 10.34 mmol, 3 equiv.) was added, followed by addition of sodium carbonate (1.10 g, 10.34 mmol, 3 equiv.); the reaction mixture was heated in 60 °C for 18 h. The following day, formation of the (S)-1-(2-aminobenzoyl)-4-(4-chlorobenzoyl)piperazine-2-carboxylic acid 9 was confirmed by LRMS-ESI spectra. The volatiles were evaporated under reduced pressure, then the residue was co-evaporated with toluene (3 × 50 mL) and dissolved in dry DMF. For cyclization of 9, HATU (3.93 g, 10.34 mmol, 3 equiv.) and DIPEA (1.80 mL, 10.34 mmol, 3 equiv.) were added, and reaction mixture was stirred in room temperature for 18 h. The day after, the volatiles were evaporated under reduced pressure and residue was dissolved in water:ethyl acetate biphasic system. The organic phase was washed with water (2 × 50 mL), 0.5 M HCl (3 × 50 mL), saturated sodium bicarbonate (1 × 50 mL), and dried over magnesium sulphate. The crude product dissolved in ethyl acetate was evaporated with silica gel (2 g) and purified by column chromatography using hexane:ethyl acetate 2:8 v/v mixture, followed by pure ethyl acetate. Yield: 711 mg (56%). 1H-NMR (500 MHz, DMSO-d6): 10.55, 10.45 (2 × s, 2 × NH); 7.80–7.70 (m, 1H, HAr); 7.70–7.40 (m, 5H, HAr); 7.30–7.20 (m, 1H, HAr); 7.20–7.00 (m, 1H, HAr); 4.45–3.30 (m, 7H, 3 × CH2, 1 × CH); 13C-NMR (125 MHz, DMSO-d6): 170.5, 169.5, 166.6, 136.6, 135.0, 134.2, 132.3, 130.9, 129.3, 129.0, 128.5, 128.3, 125.6, 124.0, 120.9, 51.7, 42.7, 42.2, 38.2; HRMS (ESI): m/z [M + H]+ calcd. for C19H17ClN3O3: 370.09530, 372.09235, found: 370.09517, 372.09206; m.p. 248–250 °C. [α]20D = +290 (c 1.0, DMSO). IR (KBr): cm−1 3465, 3369, 3229, 3160, 3109, 3068, 2909, 2866, 1694, 1657, 1620, 1521, 1477, 1431, 1407, 1339, 1303, 1262, 1219, 1181, 1162, 1092, 1035, 1010.
Molbank 2017, 2017(4), M964; doi: 10.3390/M964
Communication
(S)-2-(4-Chlorobenzoyl)-1,2,3,4-tetrahydrobenzo[e]pyrazino[1,2-a][1,4]diazepine-6,12(11H,12aH)-dione—Synthesis and Crystallographic Studies
Adam Mieczkowski 1,*, Damian Trzybiński 2, Marcin Wilczek 3, Mateusz Psurski 4 , Maciej Bagiński 1,3, Bartosz Bieszczad 1,3, Magdalena Mroczkowska 1,3 and Krzysztof Woźniak 2
, Maciej Bagiński 1,3, Bartosz Bieszczad 1,3, Magdalena Mroczkowska 1,3 and Krzysztof Woźniak 2
1
Institute of Biochemistry and Biophysics Polish Academy of Sciences, Pawinskiego 5a, 02-106 Warsaw, Poland
2
Biological and Chemical Research Centre, University of Warsaw, Żwirki i Wigury 101, 02-089 Warsaw, Poland
3
Faculty of Chemistry, University of Warsaw, Pasteura 1, 02-093 Warsaw, Poland
4
Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, 12 R. Weigl, 53-114 Wroclaw, Poland
*
Correspondence: Tel.: +48-22-592-35-06; Fax: +48-22-592-21-90
Received: 12 October 2017 / Accepted: 25 October 2017 / Published: 27 October 2017
Abstract
:
(S)-2-(4-Chlorobenzoyl)-1,2,3,4-tetrahydrobenzo[e]pyrazino[1,2-a][1,4]diazepine-6,12(11H,12aH)-dione was obtained in a three-step, one-pot synthesis, starting from optically pure (S)-2-piperazine carboxylic acid dihydrochloride. Selective acylation of the β-nitrogen atom followed by condensation with isatoic anhydride and cyclization with HATU/DIPEA to a seven-member benzodiazepine ring, led to the tricyclic benzodiazepine derivative. Crystallographic studies and initial biological screening were performed for the title compound.
Keywords:
(S)-2-piperazinecarboxylic acid; tricyclic benzodiazepines; isatoic anhydride; cytotoxicityhttp://www.mdpi.com/1422-8599/2017/4/M964/htm
file:///C:/Users/Inspiron/Downloads/molbank-2017-M964-s002.pdf
/////////
Ethyl-2-butenoate
Abstract
For the last fifty years nuclear magnetic resonance spectroscopy, generally referred as NMR, is one of the most versatile techniques for elucidation of structure of organic compounds. Among all available spectrometric methods, NMR is the only technique which offers a complete analysis and interpretation of the entire spectrum. Due to improved experimental technology and novel approaches, over the last decade nuclear magnetic resonance (NMR) has shown a tremendous progress. Generally, NMR spectroscopy makes use of three approaches; those are one dimension (1D), two dimensions (2D) and three dimensions (3D). Usually, the first approach of 1D-NMR (1H DEPT, 13C, 15N, 19F, 31P, etc.) generates good information about the structure of simple organic compounds, but in case of larger molecules the 1D-NMR spectra are generally overcrowded. Hence, the second approach of 2D-NMR (COSY, DQFCOSY, MQFCOSY, HETCOR, HSQC, HMQC, HMBC, TOCSY, NOESY, EXSY, etc.) is used for the further larger molecules, but 2D-NMR spectra also becomes complex and overlapping when used for further very large molecules like proteins. Hence, so as to achieve high resolution and reduced overlapping in spectra of very large molecules, Multi Dimensional-NMR (Homonuclear and Heteronuclear) are generally used. This paper supports interpretation of structure of different organic compounds by different NMR techniques.
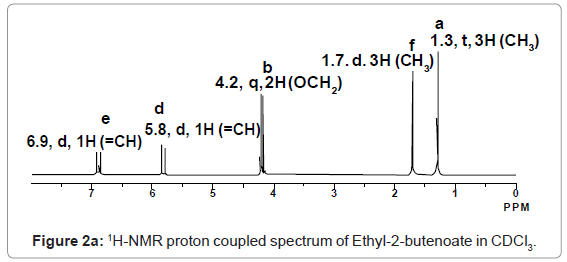
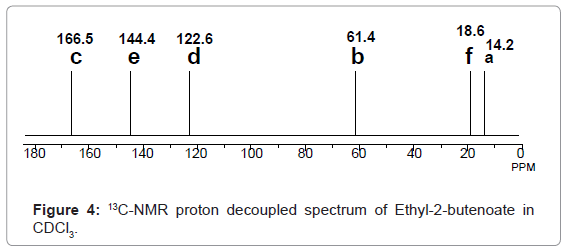
13C-NMR proton decoupled spectrum of Ethyl-2-butenoate in CDCl3.
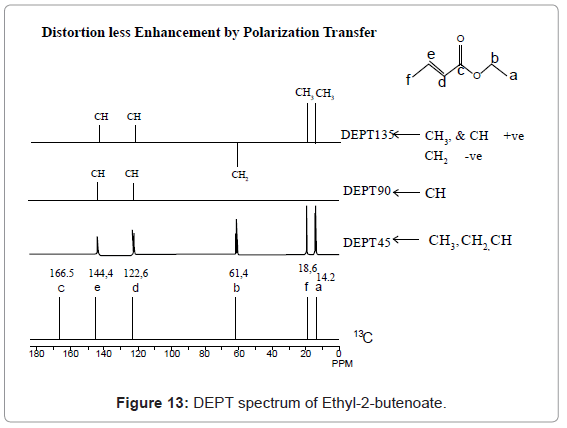
DEPT spectrum of Ethyl-2-butenoate.
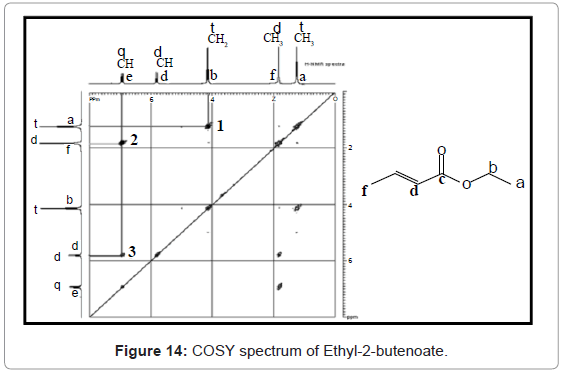
COSY
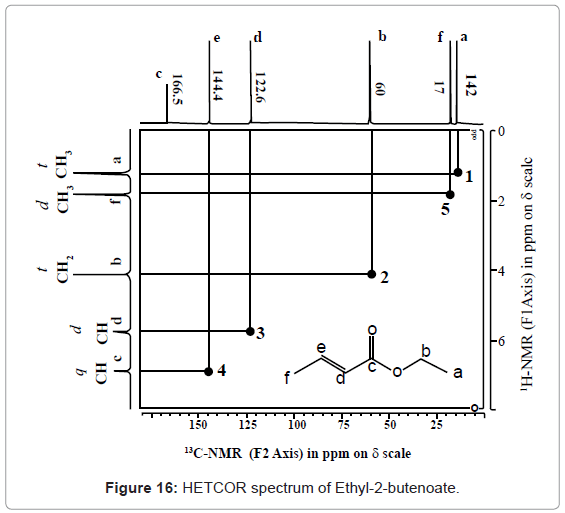
https://www.omicsonline.org/structural-elucidation-of-small-organic-molecules-by-1d-2d-and-multi-dimensional-solution-nmr-spectroscopy-2155-9872.S11-001.php?aid=12051&view=mobile
//////////
Structural Elucidation of Small Organic Molecules by 1D, 2D and Multi Dimensional-Solution NMR Spectroscopy
Neeraj Kumar Fuloria* and Shivkanya Fuloria
Anuradha College of Pharmacy, Amravati University, Maharashtra, India
- *Corresponding Author:
- Dr. Neeraj Kumar Fuloria
M.Pharm (Pharmaceutical Chemistry)
Head, M.Pharm (Quality Assurance)
Anuradha College of Pharmacy
Chikhli, Buldhana, Maharashtra, India
Tel: 8805680423
E-mail: nfuloria@gmail.com, nfuloria@rediffmail.com
Received date: January 10, 2013; Accepted date: January 30, 2013; Published date: February 07, 2013
Citation: Fuloria NK, Fuloria S (2013) Structural Elucidation of Small Organic Molecules by 1D, 2D and Multi Dimensional-Solution NMR Spectroscopy. J Anal Bioanal Tech S11:001. doi: 10.4172/2155-9872.S11-001
Copyright: © 2013 de Francisco TMG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The NMR Spectrum of Iodoethane CH3CH2I
The CH3 protons produce a peak at δ 1.8 but, instead of a single peak, a triplet is produced. This is because the CH3 protons couple with the adjacent two CH2 protons.
The CH2 protons produce a peak at δ 3.2 but, instead of a single peak, a quartet is produced. This is because the CH2 protons couple with the adjacent three CH3protons.
In general, if there are 'n' protons three bonds away from the resonating group, the absorption will be split into a multiplet of n+1 lines.
HR-MAS NMR Spectroscopy in Material Science
 Schematic of a HR-MAS stator with a magic angle gradient along the rotor spinning axis.
Schematic of a HR-MAS stator with a magic angle gradient along the rotor spinning axis. The gradient 2D 1 H HR-MAS NMR COSY spectrum for the ionic liquid [MBPyrr] + [TFSI] -

The 1 H HR-MAS NMR of the ionic liquid [MBPyrr] + TFSI - at 298K as a A) neat liquid and B)

Pictorial representation of the gradient produced along the magic angle of the rotor. B) The decay of two different water signals found in a 1N methanol solution of an AEM membrane with increasing gradient strength. Gradient strength values (G/cm) are shown above the stack plot.

//////
Subscribe to:
Comments (Atom)








