4-Hydroxy-3-methoxycinnamaldehyde (Coniferaldehyde)
C10H10O3
178.00
ESIMS m/z 178 [M – H]-
λmax 350
IR
IR(cm-¹)
(KBr) 3381 (OH),
2930 (olefinic -CH=CH-),
1675 (C=O), 1662, 1459,
1360-610 (aromatic -CH=CH-)
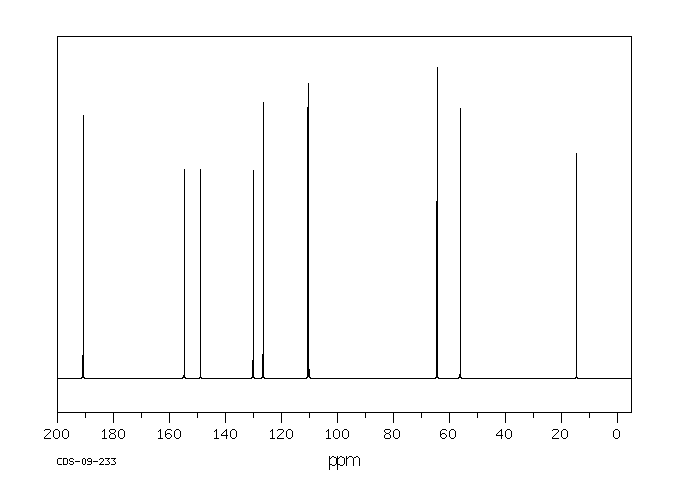
13 C NMR
(225 MHz, acetone-d6)
δ: 127.5 (C-1),
111.6 (C-2),
151.0 (C-3),
148.9 (C-4),
116.3 (C-5),
124.9 (C-6),
154.2 (C-7),
127.1 (C-8),
193.5 (C-9),
56.5 (OCH3)
1H NMR
(900 MHz, acetone-d6)
δ: 9.64 (1H, d, J = 8.1 Hz, H-9),
7.57 (1H, d, J = 16.2 Hz, H-7),
7.38 (1H, s, H-2),
7.21 (1H, d, J = 8.1 Hz, H-6),
6.91 (1H, d, J = 8.1 Hz, H-5),
6.65 (1H, dd, J = 16.2, 8.1 Hz, H-8),
3.93 (3H, s, OCH3)
1H_13C_HMBC
1H_13C_HSQC
HH_TOCSY.















 Dedicated to all moms in the world
Dedicated to all moms in the world
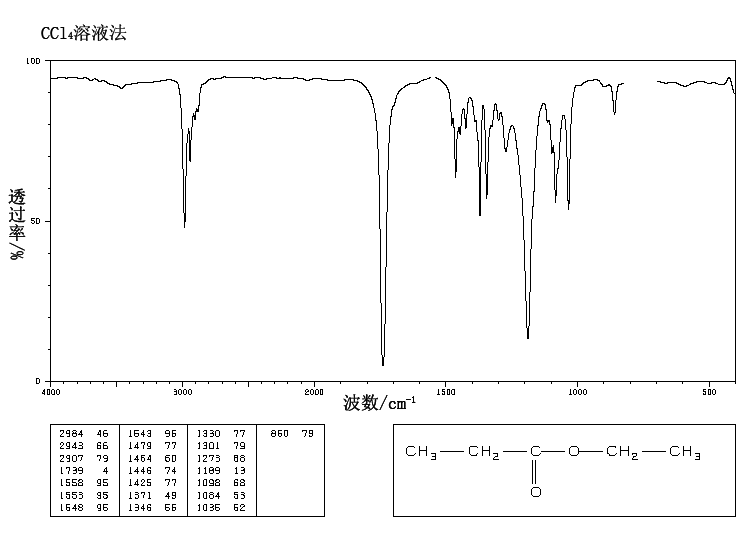
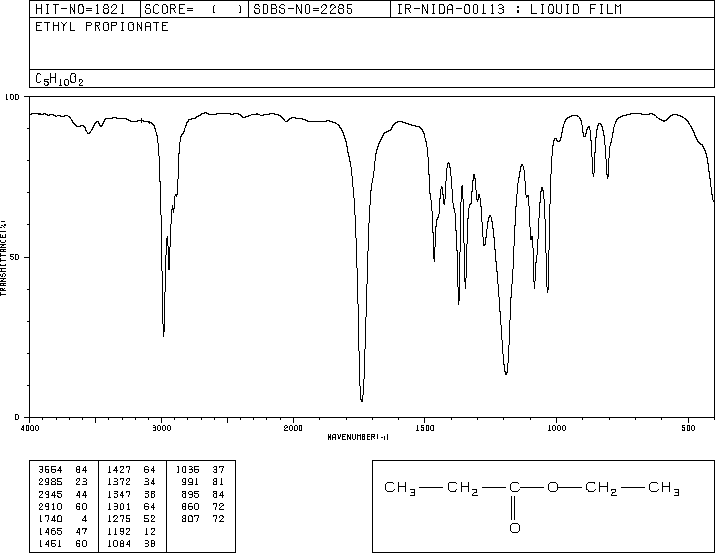


 This is the structure. See if you can assign the peaks on your own.
This is the structure. See if you can assign the peaks on your own. C has a higher chemical shift than D because it's closer to a more electron-withdrawing functional group.
C has a higher chemical shift than D because it's closer to a more electron-withdrawing functional group.