Example
C8H8OMW 120
Calculate the degree of unsaturation: the answer is 5. If the degree of unsaturation is 4 or greater, look for an aromatic ring, which has a degree of unsaturation of 4 (3 double bonds plus 1 ring). In addition to an aromatic ring, the molecule can have a carbon-carbon double bond, a carbonyl, or another ring.
IR Spectrum
Look for a carbonyl, since the degree of unsaturation indicates that the compound could have a double bond and we know that the molecule has an oxygen. There is a band at 1703, suggesting an alpha, beta unsaturated aldehyde or ketone(1710-1665). To see if the compound might be an aldehyde, look for bands in the region 2830-2695. In the spectrum below, note the two bands in this region, suggesting that the compound is indeed an aldehyde.The IR can also help determine whether or not the compound is an aromatic (although the NMR is a better diagnostic method for this). Look for the C–H stretch in aromatics from 3100-3000. Note that compounds that are not aromatic show C-H stretch from 3000-2850.
Proton NMR Spectrum
Since the IR spectrum indicates an aldehyde, look for this functionality in the NMR spectrum. The aldehydic proton appears in the NMR from 9-10, usually as a small singlet. The singlet of one proton in the spectrum below verifies the presence of an aldehyde:


Aromatic protons show up from 6.5-8.5 ppm. The four protons in the region 7.3-7.8 ppm indicate four aromatic protons; thus the aromatic ring has two substituents. Benzylic protons - hydrogens on a carbon adjacent to an aromatic ring - show up from 2-3 ppm; the three protons in the singlet peak at 2.4 ppm are likely benzylic protons. Since there are three protons, it is a methyl group.
Therefore we know that the molecule is a di-substituted aromatic ring with a -CHO group and a -CH3 group. Double check that we have the proper number of carbons: 6 (in ring) plus 1 (aldehyde) plus 1 (methyl) equals 8, the number given in the molecular formula.
Therefore we know that the molecule is a di-substituted aromatic ring with a -CHO group and a -CH3 group. Double check that we have the proper number of carbons: 6 (in ring) plus 1 (aldehyde) plus 1 (methyl) equals 8, the number given in the molecular formula.
These hydrogens correspond as follows with the peaks in the NMR:
The molecule is 4-methylbenzaldehyde: a para substituted aromatic. In the organic chemistry teaching laboratory courses, you are not held responsible for proper assignment of ortho-, meta-, or para-substituted rings. However, para substituted rings almost always show a symmetrical pattern in the aromatic region, as in the spectrum above. (See Below
13 C NMR
WATCH OUT FOR INTERPRETATION OF 13 C NMR
Summary and answer
Example is 4-methylbenzaldehyde:MORE
IR Spectroscopy
Aromatics show a lot more bands in an IR spectrum than do alkanes, alkenes, and alkynes. The C-C stretching vibrations show up as several bands in the region 1600-1585 and 1500-1400.
One of the most “telling” bands is the presence of a band just to the left of 3000. Alkyl groups show up to the right of 3000, aromatic C–H stretches to the left of 3000.
NMR SpectroscopyOne of the most “telling” bands is the presence of a band just to the left of 3000. Alkyl groups show up to the right of 3000, aromatic C–H stretches to the left of 3000.
- C–H stretch from 3100-3000
- overtones, weak, from 2000-1665
- C–C stretch (in-ring) from 1600-1585
- C–C stretch (in-ring) from 1500-1400
- C–H “oop” from 900-675
Aromatic protons show up from 6.5-8.5 ppm. Benzylic protons are from 2–3 ppm.
If an aromatic ring has more than one substituent, careful analysis of the shifts and splitting pattern of the protons in the aromatic region reveals the positions on the ring of the different substituents. However, the the shifts depend on the substituents and the splitting is not really first order. Most sophomore-level courses do not cover NMR spectroscopy in the depth required to analyze the aromatic region. Therefore, you will not be able to designate the exact ring substition in many cases.
In the specific case of disubstituted aromatic rings, para-substituted rings usually show two symmetric sets of peaks that look like doublets. The para-substitution NMR aromatic region pattern usually looks quite different than the patterns for both ortho- and meta-substituted aromatic rings.
Examples of ortho, meta, and para substitution are illustrated in the NMR spectra of different isomers of chloronitrobenzene, below. The CDCl3 peak is pointed out in each spectrum. (The samples were run using CDCl3as the solvent, and a small contaminant of this deuterated solvent is CHCl3, which shows up at 7.24 ppm. This is used to calibrate the spectrum.) Note the symmetry of the para substituted chloronitro benzene. (Click on each full-size image to view details of the region from 6.5-8.5 ppm.)
SOME INTERESTING INFO
Trimethyl 2-Hydroxy-2-(2-methoxy-2-oxoethyl)-4-(4-methylphenyl)-6-oxo-1,3,5-cyclohexanetricarboxylate
Edmont V. Stoyanov
Faculty of Pharmacy, Medical University of Sofia, Dunav 2, BG-1000 Sofia, Bulgaria
Tel.: (+359 2) 988 3142; Fax: (+359 2) 987 9874; E-mail: estoyanov@yahoo.com
Tel.: (+359 2) 988 3142; Fax: (+359 2) 987 9874; E-mail: estoyanov@yahoo.com
Aromatic aldehydes react with dimethyl acetonedicarboxylate in molar ratio 1:2 with spontaneous intermolecular Michael addition to give polysubstituted cyclohexanones [1]. We report now the synthesis of an analogous product from 4-methylbenzaldehyde.
To a solution of 4-methylbenzaldehyde (1.20 g, 10 mmol) and dimethyl acetonedicarboxylate (3.48 g, 20 mmol) in 25 ml ethanol, 0.3 ml piperidine was added. The reaction mixture was left to stay at room temperature for 3 days. The separated crystals were filtered off, washed with cold ethanol, recrystallized from dioxane and air-dried. Yield: 3.18 g (71 %).
Colorless crystals, m. p. 149-150 ºC (dec.) from dioxane.
1H NMR (300 MHz, d6-DMSO):
2.23 (s, 3H, PhCH3), 2.43 (d, 1H, J=17.0 Hz, HCH),
2.96 (d, 1H, J=17.0 Hz, HCH), 3.44 (s, 3H, OCH3),
3.52 (d, 1H, J=12.2 Hz, H-3), 3.56 (s, 3H, OCH3),
3.60 (s, 3H, OCH3), 3.66 (s, 3H, OCH3),
3.94 (t, J1=J2=12.2 Hz, H-4), 4.38 (d, 1H, J=12.2 Hz, H-5),
4.67 (s, 1H, H-1), 5.40 (s, 1H, OH),
7.06 (d, 2H, J=8.0 Hz, 2' and 6'H arom.),
7.19 (d, 2H, J=8.0 Hz, 3' and 5'H arom.).
2.23 (s, 3H, PhCH3), 2.43 (d, 1H, J=17.0 Hz, HCH),
2.96 (d, 1H, J=17.0 Hz, HCH), 3.44 (s, 3H, OCH3),
3.52 (d, 1H, J=12.2 Hz, H-3), 3.56 (s, 3H, OCH3),
3.60 (s, 3H, OCH3), 3.66 (s, 3H, OCH3),
3.94 (t, J1=J2=12.2 Hz, H-4), 4.38 (d, 1H, J=12.2 Hz, H-5),
4.67 (s, 1H, H-1), 5.40 (s, 1H, OH),
7.06 (d, 2H, J=8.0 Hz, 2' and 6'H arom.),
7.19 (d, 2H, J=8.0 Hz, 3' and 5'H arom.).
13C NMR (75 MHz, d6-DMSO):
41.4, 42.8, 51.1, 51.5, 51.6, 51.7, 54.3, 61.2, 62.8, 66.3, 74.3,
128.1 (2xC), 128.9 (2xC), 136.2,
136.6 (2xC), 167.7, 168.2, 169.5, 169.8.
41.4, 42.8, 51.1, 51.5, 51.6, 51.7, 54.3, 61.2, 62.8, 66.3, 74.3,
128.1 (2xC), 128.9 (2xC), 136.2,
136.6 (2xC), 167.7, 168.2, 169.5, 169.8.
FT IR (KBr, cm-1): 3511, 2953, 1729, 1516, 1495, 1364.
ESI MS [FIA in MeOH, CH3COONH4/CH3COOK]: 468.2 [M+NH4]+, 489.2 [M+K]+.
Reference
1. Haensel, W.; Haller, R. Arch. Pharm. (Weinheim Ger.) 1970, 303, 334-338.
lahore, pakistan
Punjabi sweets on a leaf in Lahore Pakistan, 20 Rupees or about 30 cents. I wasn't sure if one is supposed to eat the leaf too but I ate it anyways. 10/07
New Food Street Lahore
..............
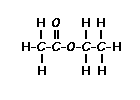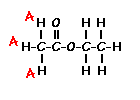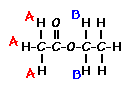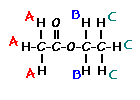
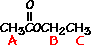


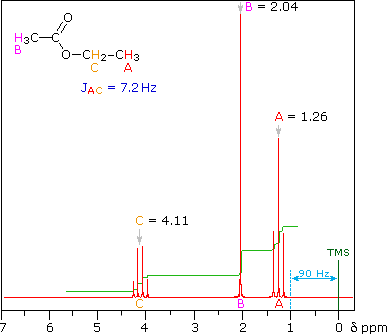
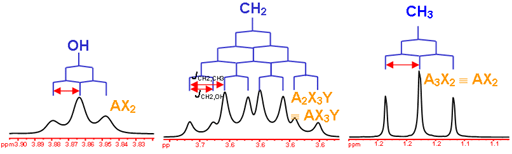



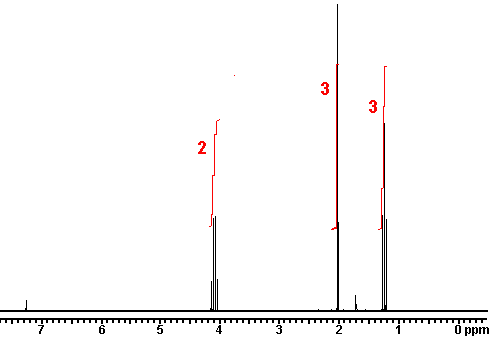
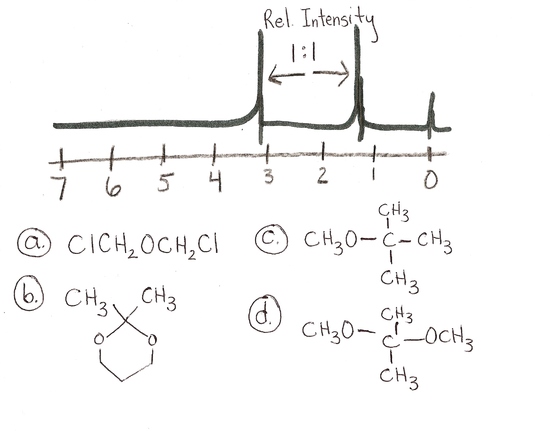 answer is a and d
answer is a and d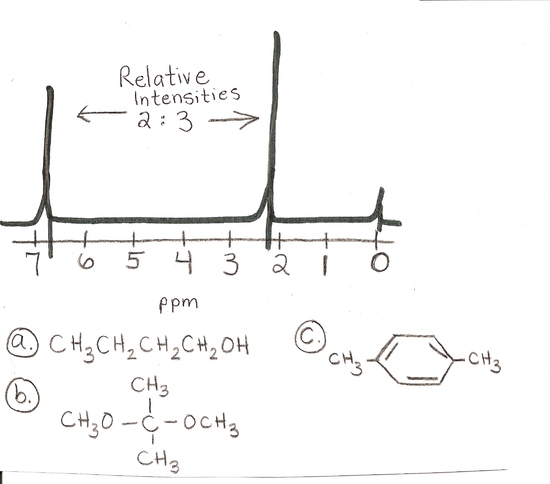
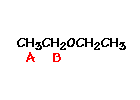
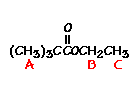




 n
n



