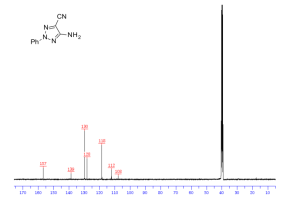
Carbon-13 NMR (13C NMR or sometimes simply referred to as carbon NMR) is the application of nuclear magnetic resonance (NMR) spectroscopy to carbon. It is analogous to proton NMR (1H NMR) and allows the identification of carbon atoms in an organic moleculejust as proton NMR identifies hydrogen atoms. As such 13C NMR is an important tool in chemical structure elucidation in organic chemistry. 13C NMR detects only the 13C isotope of carbon, whose natural abundance is only 1.1%, because the main carbon isotope,12C, is not detectable by NMR since it has zero net spin.
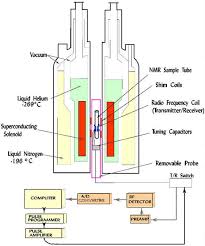
Implementation
13C NMR has a number of complications that are not encountered in proton NMR. 13C NMR is much less sensitive to carbon than 1H NMR is to hydrogen since the major isotope of carbon, the 12C isotope, has a spin quantum number of zero and so is not magnetically active and therefore not detectable by NMR. Only the much less common 13C isotope, present naturally at 1.1% natural abundance, is magnetically active with a spin quantum number of 1/2 (like 1H) and therefore detectable by NMR. Therefore, only the few 13C nuclei present resonate in the magnetic field, although this can be overcome by isotopic enrichment of e.g. protein samples. In addition, thegyromagnetic ratio (6.728284 107 rad T−1 s−1) is only 1/4 that of 1H, further reducing the sensitivity. The overall receptivity of 13C is about 4 orders of magnitude lower than 1H.
[1]

[1]
Another potential complication results from the presence of large one bond J-coupling constants
between carbon and hydrogen (typically from 100 to 250 Hz). In order to
suppress these couplings, which would otherwise complicate the spectra
and further reduce sensitivity, carbon NMR spectra are proton decoupled to remove the signal splitting. Couplings between carbons can be ignored due to the low natural abundance of 13C.
Hence in contrast to typical proton NMR spectra which show multiplets
for each proton position, carbon NMR spectra show a single peak for each
chemically non-equivalent carbon atom.
In further contrast to 1H NMR, the intensities of the signals are not normally proportional to the number of equivalent 13C atoms and are instead strongly dependent on the number of surrounding spins (typically 1H). Spectra can be made more quantitative if necessary by allowing sufficient time for the nuclei to relax between repeat scans.


High field magnets with internal bores capable of accepting larger sample tubes (typically 10 mm in diameter for 13C NMR versus 5 mm for 1H NMR), the use of relaxation reagents,[2] for example Cr(acac)3 (chromium
(III) acetylacetonate, CAS number 21679-31-2), and appropriate pulse
sequences have reduced the time needed to acquire quantitative spectra
and have made quantitative carbon-13 NMR a commonly used technique in
many industrial labs. Applications range from quantification of drug purity to determination of the composition of high molecular weight synthetic polymers.
13C chemical shifts follow the same principles as those of 1H, although the typical range of chemical shifts is much larger than for 1H (by a factor of about 20). The chemical shift reference standard for 13C is the carbons in tetramethylsilane (TMS), whose chemical shift is considered to be 0.0 ppm.
Typical chemical shifts in 13C-NMR
DEPT spectra
DEPT stands for Distortionless Enhancement by Polarization Transfer. It is a very useful method for determining the presence of primary, secondary andtertiary carbon atoms. The DEPT experiment differentiates between CH, CH2and CH3 groups by variation of the selection angle parameter (the tip angle of the final 1H pulse):
- 135° angle gives all CH and CH3 in a phase opposite to CH2
- 90° angle gives only CH groups, the others being suppressed
- 45° angle gives all carbons with attached protons (regardless of number) in phase
Signals from quaternary carbons and other carbons with no attached protons are always absent (due to the lack of attached protons).
The polarization transfer from 1H to 13C has the secondary advantage of increasing the sensitivity over the normal 13C spectrum (which has a modest enhancement from the NOE (Nuclear Overhauser Effect) due to the 1H decoupling).
APT spectra
Another useful way of determining how many protons a carbon in a molecule is bonded to is to use an Attached Proton Test, which distinguishes between carbon atoms with even or odd number of attached hydrogens. A proper spin-echo sequence is able to distinguish between S, I2S and I1S, I3S
spin systems: the first will appear as positive peaks in the spectrum,
while the latter as negative peaks (pointing downwards), while retaining
relative simplicity in the spectrum since it is still broadband proton
decoupled.
Even though this technique does not distinguish fully between CHn groups,
it is so easy and reliable that it is frequently employed as a first
attempt to assign peaks in the spectrum and elucidate the structure.[3]
- ^ R. M. Silverstein, G. C. Bassler and T. C. Morrill (1991). Spectrometric Identification of Organic Compounds. Wiley.
- ^ Caytan, Elsa; Remaud, Gerald S.; Tenailleau, Eve; Akoka, Serge, GS; Tenailleau, E; Akoka, S (2007). "Precise and accurate quantitative 13C NMR with reduced experimental time". Talanta 71 (3): 1016–1021. doi:10.1016/j.talanta.2006.05.075. PMID 19071407
- ^ Keeler, James (2010). Understanding NMR Spectroscopy (2nd ed.). John Wiley & Sons. p. 457. ISBN 978-0-470-74608-0.
- Carbon NMR spectra, where there are three spectra of ethyl phthalate, ethyl ester of orthophthalic acid: completely coupled, completely decoupled and off-resonance decoupled (in this order).
- For an extended tabulation of 13C shifts and coupling constants.












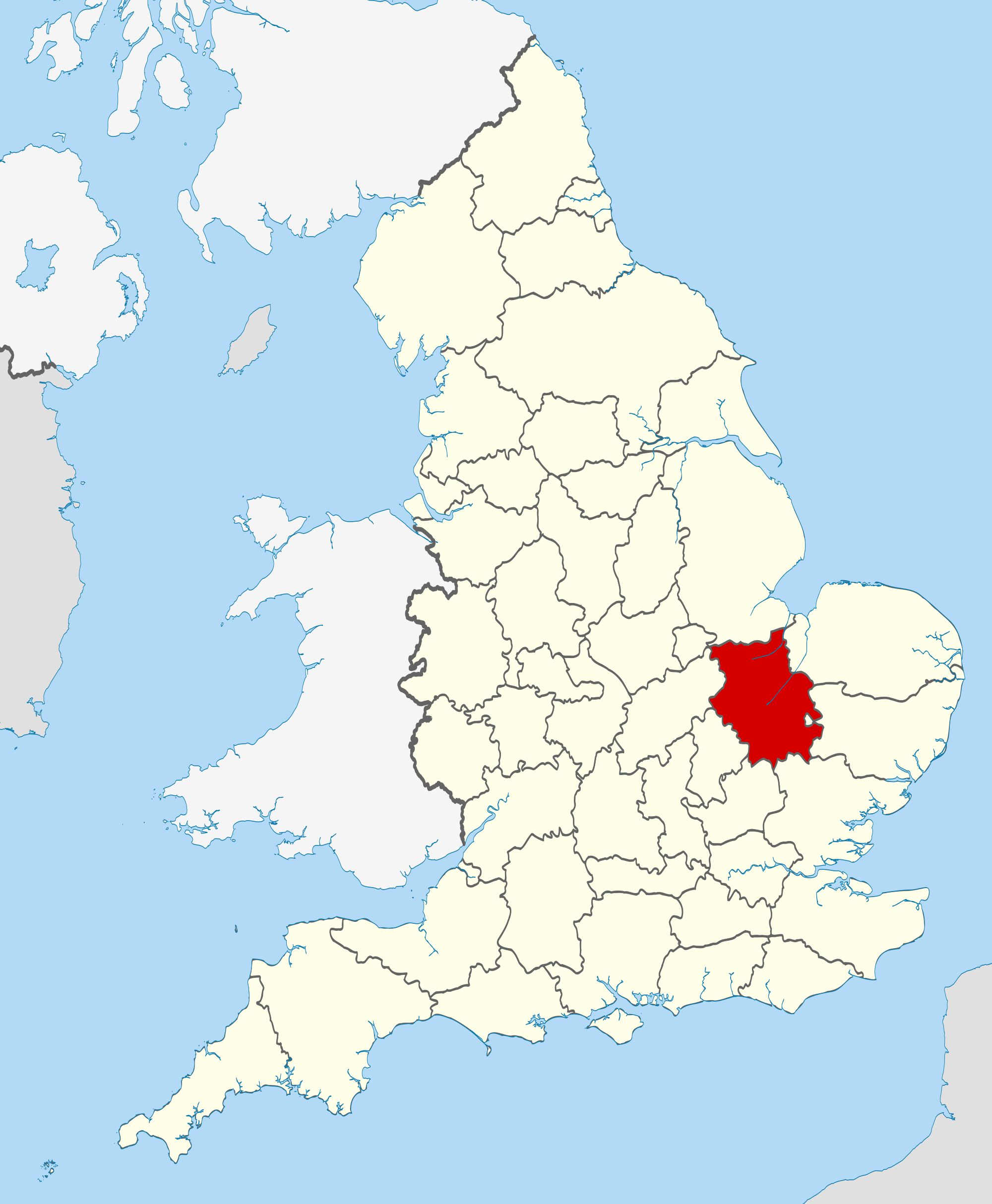




 Halifax survey names South Cambridgeshire as best place to live in rural Britain
Halifax survey names South Cambridgeshire as best place to live in rural Britain