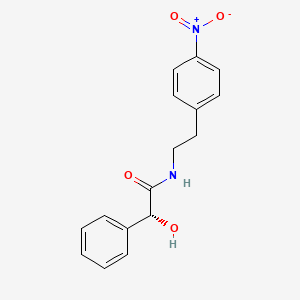
\\
white solid
(2.97 g, 64%). Rf = 0.25 (pentane/EtOAc, 90:10);
IR (neat) νmax 3082, 2946, 1736, 1731,
1504 cm-1
;
M.P. = 35-39 °C;
1H NMR (500 MHz, CDCl3) δ 7.30-7.26 (m, 1H), 7.22 (dd, J =
8.1, 1.3 Hz, 1H), 7.12 (td, J = 7.5, 1.2 Hz, 1H), 7.07 (dd, J = 8.2, 1.2 Hz, 1H), 5.83 (ddt, J =
16.8, 10.3, 5.7 Hz, 1H), 5.28-5.24 (m, 1H), 5.23-5.20 (m, 1H), 4.65 (qdt, J = 13.2, 5.8, 1.4
Hz, 2H), 3.80 (dd, J = 8.8, 6.1 Hz, 1H), 3.44 (dd, J = 16.0, 8.8 Hz, 1H), 3.19 (dd, J = 16.0,
6.1 Hz, 1H);
13C NMR (126 MHz, CDCl3) δ 167.2, 164.6, 151.4, 131.0, 128.7, 128.3, 124.8,
120.6, 118.9, 116.9, 66.5, 46.4, 27.3;
HRMS: (ESI-TOF) calculated for C13H12O4 [M+
]
232.0736, found 232.0731.
////////////////