Laura Beswick
2nd degree connection
PhD Student at Keele University
Gavin J. Miller
2nd degree connection
Lecturer in Organic Chemistry at Keele University
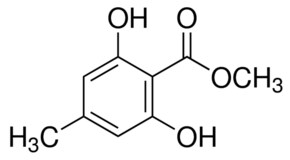
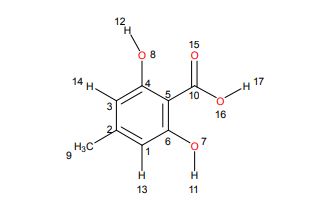
3.4. 1,2,3,4-Tetra-O-Acetyl-β-d-Mannuronic Acid (4)
To a vigorously stirred solution of 1,2,3,4-tetra-O-acetyl-β-d-mannopyranose (3) (1.5 g, 4.30 mmol, 1.0 equiv.) in dichloromethane (20 mL) and water (10 mL) was added (2,2,6,6-tetramethylpiperidin-1-yl)oxyl radical (TEMPO) (336 mg, 2.2 mmol, 0.50 equiv.) and [bis(acetoxy)iodo]benzene (BAIB) (6.9 g, 21.5 mmol, 5.00 equiv.). The solution was stirred at RT for 5 h, whereupon TLC analysis (hexane/ethyl acetate, 1/1) showed complete conversion of starting material to a baseline Rf value spot. The reaction was quenched with aqueous Na2S2O3 solution (10% w/v, 30 mL), the organic layer removed and the aqueous layer acidified to pH 3 using 1M HCl, followed by extraction with ethyl acetate (2 × 30 mL). The organic extracts were combined, dried (MgSO4), filtered and concentrated in vacuo to afford 1,2,3,4-tetra-O-acetyl-β-d-mannuronic acid (4) as a white solid (1.2 g, 3.3 mmol, 75%). Rf = 0.81 (dichloromethane/methanol, 9/1);
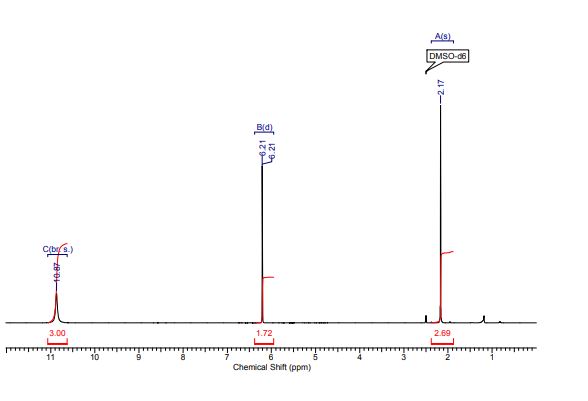
1H-NMR (300 MHz, CDCl3) δH5.96 (1H, d, J = 1.5 Hz, H1), 5.50 (1H, dd, J = 3.2, 1.5 Hz, H2), 5.48 (1H, appt, J = 9.1 Hz, H4), 5.21 (1H, dd, J = 9.1, 3.2 Hz, H3), 4.2 (1H, d, J = 9.1 Hz, H5), 2.21 (3H, s, C(O)CH3), 2.13 (3H, s, C(O)CH3), 2.10 (3H, s, C(O)CH3), 2.04 (3H, s, C(O)CH3).
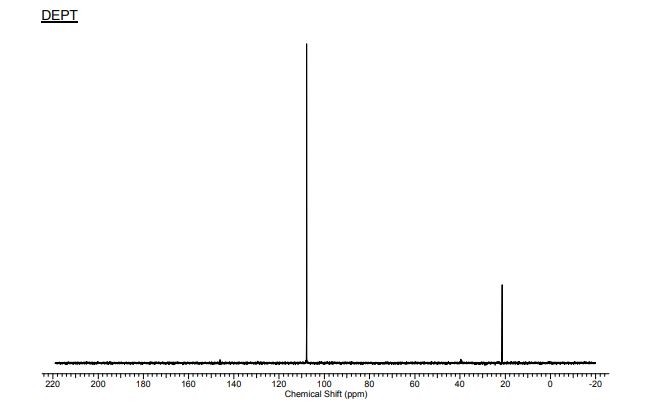
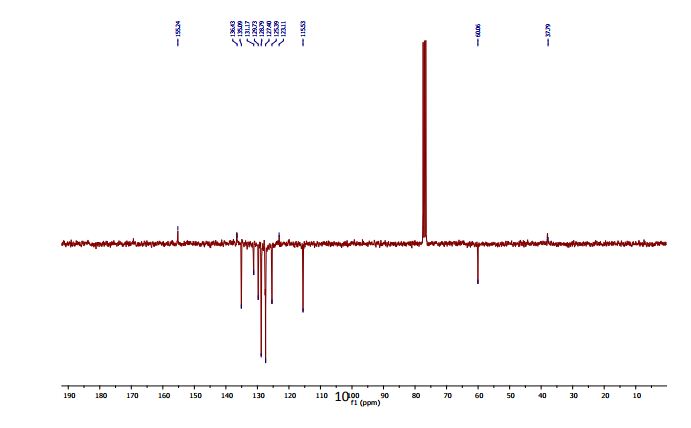
13C-NMR (100 MHz, CDCl3) δC 169.9 (C=O), 169.7 (C=O), 169.5 (C=O), 169.4 (C=O), 168.2 (C=O), 89.4 (C1), 72.3 (C5), 69.3 (C3), 66.9 (C2), 65.9 (C4), 20.4 (C(O)CH3), 20.4 (C(O)CH3), 20.3 (C(O)CH3), 20.2 (C(O)CH3);
FT-HRMS NSI (ES−) m/z Found: (M−H)− 361.0767, C14H17O11 requires 361.0776; FT-IR vmax/cm−1 2986 (O–H), 1751 (C=O).
Communication
1,2,3,4-Tetra-O-Acetyl-β-d-Mannuronic Acid
Laura Beswick and Gavin J. Miller
*
Lennard-Jones Laboratory, School of Chemical and Physical Sciences, Keele University, Keele, Staffordshire ST5 5BG, UK
*
Correspondence: Tel.: +44-1782-734442
Received: 3 July 2017 / Accepted: 11 July 2017 / Published: 14 July 2017
Abstract
:
1,2,3,4-Tetra-O-acetyl-β-d-mannuronic acid was synthesized in three steps from commercial d-mannose in 21% yield. Regioselective 6-O-tritylation followed by per-acetylation and 6-OTr removal using HBr/AcOH gave the required primary alcohol substrate, which was then oxidised to the target compound using TEMPO/BAIB. None of the synthetic steps required column chromatography and the product was fully characterized by 1H-NMR, 13C-NMR, 2D NMR, MS and IR.
Keele University, Keele, Staffordshire
Lennard-Jones Laboratory, School of Chemical and Physical Sciences, Keele University,
“ORGANIC SPECTROSCOPY INT” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This is a compilation for educational purposes only. P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent