1H NMR
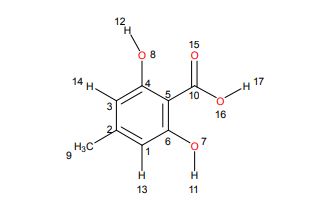
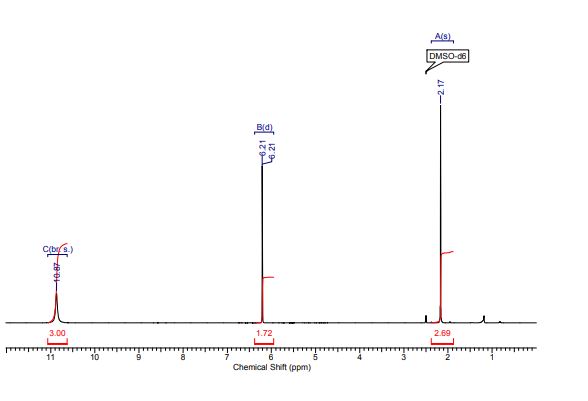
1H NMR (298.2 K, DMSO-d6, 300 MHz, δ in ppm): C 10.87 (br. s, 3 H, H17,12,11), B 6.21 (d, 2 H, J = 0.57 Hz, H14,13), A 2.17 (s, 3 H, H9).
13C NMR

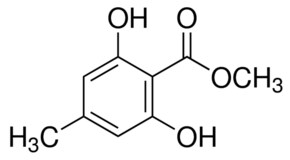
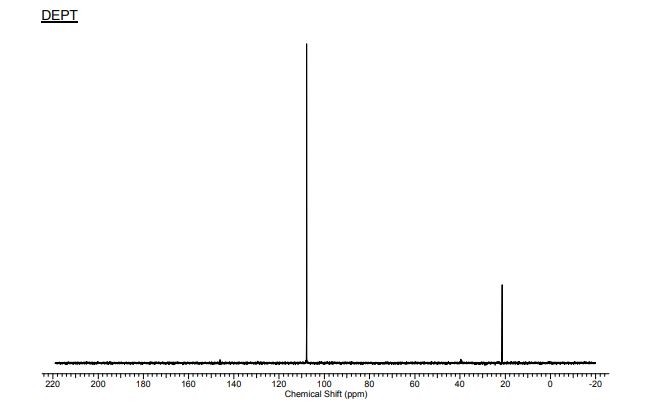
13C NMR (298.2 K, DMSO-d6, 300 MHz, δ in ppm): 9 21.57(C9), 2 98.95 (C2), 1/3 107.98 (C1,3). ), 5 146.29 (C5), 4/6 160.68 (C4,6), 10 172.31 (C10).

In this case study the regioselective enzymatic carboxylation of 3,5-dihydroxytoluene (orcinol) using the nonoxidative 2,3-dihydroxybenzoic acid decarboxylase from Aspergillus oryzae (2,3-DHBD_Ao), followed by an adsorbent-based downstream approach, has been investigated. The product 2,6-dihydroxy-4-methylbenzoic acid (DHMBA) was herein purified by an adsorption–desorption cycle and subsequently obtained with purities >99% without a full elimination of the excess bicarbonate from its reaction solution. Ten adsorbent resins were studied in respect of their ability to recover the product from the reaction solution, whereas the strong anion exchange resin Dowex 1x2 in its chloride form showed affinities >99%, even at bicarbonate concentrations of >3 mol·L–1. Desorption from loaded resin was carried out by a 2 mol·L–1 HCl/acetone mixture, followed by product crystallization during acetone evaporation. This presented concept does not require a final column preparation step and improves the overall atom efficiency of the biocatalytic reaction system.
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.8b00104
//////////////
No comments:
Post a Comment