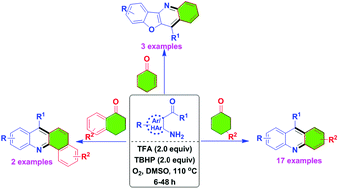
Silver-initiated radical ring expansion/fluorination of ethynyl cyclobutanols: efficient synthesis of monofluoroethenyl cyclopentanones
Silver-initiated radical ring expansion/fluorination of ethynyl cyclobutanols: efficient synthesis of monofluoroethenyl cyclopentanones
*
Corresponding authors
a
State Key Laboratory of Organometallic Chemistry Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, P. R. China
E-mail: guozhuzhang@sioc.ac.cn
E-mail: guozhuzhang@sioc.ac.cn
Green Chem., 2016, Advance Article
DOI: 10.1039/C6GC02656G
A stereoselective synthesis of β-halogenated 2-methylenecyclopentanones via silver-catalyzed formal ring expansion using water as the cosolvent is described. A variety of 2-methylenecyclopentanones with fluoro, chloro and bromo functionalities are efficiently prepared from 1-alkynyl cyclobutanols. This method offers facile access to halogenated complex molecules which are not only useful chemicals but also valuable building blocks for further derivatizations.
1-(m-tolylethynyl)cyclobutan-1-ol (1b) Yield: 89%; Yellow oil;
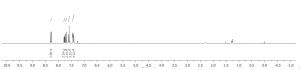
1H NMR (400 MHz, CDCl3)
δ 7.25 (s, 1H), 7.23 (d, J = 8.1 Hz, 1H), 7.18 (t, J = 7.5 Hz, 1H), 7.10 (d, J = 7.5 Hz, 1H), 2.51 (dt, J = 15.8, 6.3 Hz, 2H), 2.34 (t, J = 9.3 Hz, 2H), 2.30 (s, 3H), 1.85 (m, 2H);
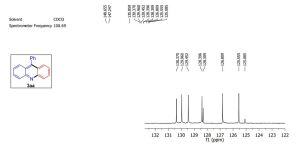
13C NMR (100 MHz, CDCl3)
δ 137.94, 132.27, 129.19, 128.72, 128.17, 122.49, 92.16, 83.58, 68.31, 38.64, 21.20, 12.98; HRMS (EI+ , 70 eV): C13H14O [M]+ : calcd. 186.1045, found 186.1047
////////