Green Chem., 2016, Advance Article
DOI: 10.1039/C6GC02396G, Communication
Gopal Chandru Senadi, Ganesh Kumar Dhandabani, Wan-Ping Hu, Jeh-Jeng Wang
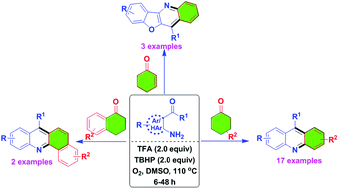
We have identified metal-free reaction conditions for the annulation/aerobic oxidative dehydrogenation of cyclohexanones with o-acylanilines to the corresponding acridine derivatives.
We have identified metal-free reaction conditions for the annulation/aerobic oxidative dehydrogenation of cyclohexanones with o-acylanilines to the corresponding acridine derivatives.
Metal-free annulation/aerobic oxidative dehydrogenation of cyclohexanones with o-acylanilines: efficient syntheses of acridines
Metal-free annulation/aerobic oxidative dehydrogenation of cyclohexanones with o-acylanilines: efficient syntheses of acridines
*Corresponding authors
aDepartment of Medicinal and Applied Chemistry, Kaohsiung Medical University, No. 100, Shiquan 1st Rd, Sanmin District, Kaohsiung City, Taiwan
E-mail: jjwang@kmu.edu.tw
E-mail: jjwang@kmu.edu.tw
bDepartment of Biotechnology, Kaohsiung Medical University, No. 100, Shiquan 1st Rd, Sanmin District, Kaohsiung City, Taiwan
Green Chem., 2016, Advance Article
DOI: 10.1039/C6GC02396G, http://pubs.rsc.org/en/Content/ArticleLanding/2016/GC/C6GC02396G?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract



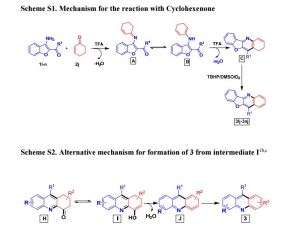
We have identified metal-free reaction conditions for the annulation/aerobic oxidative dehydrogenation of cyclohexanones with o-acylanilines to the corresponding acridine derivatives. The combination of trifluoroacetic acid (TFA), tert-butyl hydroperoxide (TBHP), dimethylsulfoxide (DMSO) and oxygen (O2) converted the cyclohexanone derivatives to an aromatic aryl product in moderate to good yields. The experimental results suggest that this process involves an aza-allyl oxidation intermediate.

Prof. Jeh-Jeng Wang
Professor
Department of Medicinal and Applied Chemistry
Kaohsiung Medical University
Kaohsiung, Taiwan
Department of Medicinal and Applied Chemistry
Kaohsiung Medical University
Kaohsiung, Taiwan
Lab: N842, Bioorganic chemistry Laboratory
Email: jjwang@kmu.edu.tw
Tel: +886-7-3121101
Fax: 886-7-3125339
Click here to see my official Faculty page at KMU
Email: jjwang@kmu.edu.tw
Tel: +886-7-3121101
Fax: 886-7-3125339
Click here to see my official Faculty page at KMU
Prof. Jeh-Jeng Wang
Education:
B.S., Chemistry, National Chung-Hsing University, Taiwan (1975-1979) Ph.D., Chemistry, The Ohio State University, USA (1983-1989) Career: Teaching Assistant, National Chung-Hsing University, Taiwan (1981-1983) Postdoctoral fellow, College of Pharmacy, University of Texas, Austin, USA (1989-1991) Associate Professor, Department of Medicinal and Applied Chemistry, Kaohsiung Medical University, Taiwan (1991-2001) Professor, Department of Medicinal and Applied Chemistry, Kaohsiung Medical University, Taiwan (since 2001) |
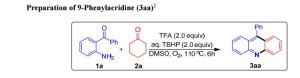
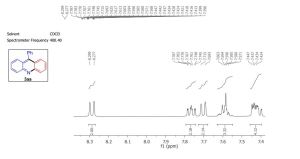
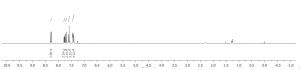
3aa as a pale yellow solid (234 mg, 92%); m.p. 181-182 ° C; IR (neat)max: 1362 cm -1; 1 H NMR (400 MHz, CDCl3) δ 8.31 (d, J = 8.8 Hz, 2H), 7.76 (ddd, J = 8.0, 6.8, 1.6 Hz, 2H), 7.72 (d, J = 8.8 Hz, 2H), 7.66 – 7.56 (m, 3H), 7.49 – 7.41 (m, 4H);
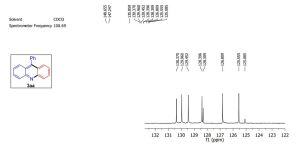
13C NMR (101 MHz, CDCl3) δ 148.66, 147.24, 135.85, 130.37, 129.96, 129.45, 128.40, 128.31, 12
1H NMR
13 c nmr

//////////Metal-free annulatio, erobic oxidative dehydrogenation, cyclohexanones, o-acylanilines, acridines

Prof. J.J. Wang birthday party - October 21, 2016
Department of Medicinal and Applied Chemistry
Kaohsiung Medical University


Kaohsiung, TAIWAN




Department of Medicinal and Applied Chemistry
Kaohsiung Medical University
Kaohsiung, TAIWAN





////////


No comments:
Post a Comment