doxercalciferol
- Molecular FormulaC28H44O2
- Average mass412.648
доксэркальциферол [Russian]
دوكساركالسيفيرول [Arabic]
度骨化醇 [Chinese]
1,3-Cyclohexanediol
54573-75-0
Title: Doxercalciferol
CAS Registry Number: 54573-75-0
CAS Name: (1a,3b,5Z,7E,22E)-9,10-Secoergosta-5,7,10(19),22-tetraene-1,3-diol
Additional Names: 1a-hydroxyvitamin D2; 1-hydroxyergocalciferol
Trademarks: Hectorol (Bone Care)
Molecular Formula: C28H44O2
Molecular Weight: 412.65
Percent Composition: C 81.50%, H 10.75%, O 7.75%
Literature References: Synthetic vitamin D prohormone. Prepn: H.-Y. P. Lam et al., Science 186, 1038 (1974); eidem, Steroids30, 671 (1977); H. E. Paaren et al., J. Org. Chem. 45, 3253 (1980). Comparative activity and toxicity: G. Sjöden et al., Proc. Soc. Exp. Biol. Med. 178, 432 (1985). Metabolism to bioactive form: J. C. Knutson et al., Endocrinology 136, 4749 (1995). Pharmacology: J. W. Coburn et al., Nephrol. Dial. Transplant. 11, Suppl. 3, 153 (1996). Clinical trial for suppression of secondary hyperparathyroidism in hemodialysis: J. M. Frazao et al., ibid. 13, Suppl. 3, 68 (1998).
Properties: Crystals, mp 138-140°. uv max (ethanol): 265 nm (e 18300). LD50 orally in rats: 3.5-6.5 mg/kg (Sjöden).
Melting point: mp 138-140°
Absorption maximum: uv max (ethanol): 265 nm (e 18300)
Toxicity data: LD50 orally in rats: 3.5-6.5 mg/kg (Sjöden)
Therap-Cat: Antihyperparathyroid.
Keywords: Antihyperparathyroid.
|

CLIP
Doxercalciferol (1α-hydroxyvitamin D2) is a commercially approved vitamin D derivative used to treat chronic kidney disease (CKD) patients whose kidneys cannot metabolically introduce a hydroxyl group at C1. A new process for the production of doxercalciferol from ergocalciferol was developed using a continuous photoisomerization of a known vitamin D intermediate as the key step, thus circumventing the limitations of batch photoisomerization processes. Doxercalciferol is produced in an overall yield of about 10% from ergocalciferol.
Doxercalciferol
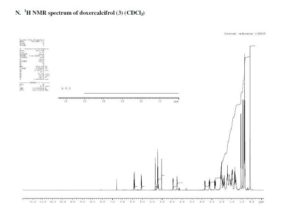
1H NMR (CDCl3) δ 6.40 (d, 1H, J = 11.2), 6.04 (d, 1H, J = 11.2), 5.35 (s, 1H), 5.15–5.29 (m, 2H), 5.03 (s, 1H), 4.45 (dd, 1H, J = 7.3, 4.0), 4.21–4.31 (m, 1H), 2.81–2.90 (m, 1H), 2.62 (d, 1H, J = 13.3), 2.34 (dd, 1H, J = 13.3, 6.5), 1.83–2.11(m, 6H), 1.42–1.79 (m, 7H), 1.21–1.40 (m, 3H), 1.04 (d, 3H, J = 6.6), 0.94 (d, 3H, J = 6.8), 0.86 (t, 6H, J = 7.3), 0.58 (s, 3H) ppm.
Doxercalciferol (trade name Hectorol) is drug for secondary hyperparathyroidism and metabolic bone disease.[1] It is a synthetic analog of ergocalciferol (vitamin D2). It suppresses parathyroid synthesis and secretion.[2]
PATENT

CLIP
References
 | |
| Names | |
|---|---|
| IUPAC name
(1S,3R,5Z,7E,22E)-9,10-Secoergosta-5,7,10,22-tetraene-1,3-diol
| |
| Other names
1-Hydroxyergocalciferol; 1-Hydroxyvitamin D2; 1α-Hydroxyergocalciferol; 1α-Hydroxyvitamin D2; Hectorol; TSA 840
| |
| Identifiers | |
| 54573-75-0 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL1200810 |
| ChemSpider | 4444554 |
| DrugBank | DB06410 |
| ECHA InfoCard | 100.170.997 |
| 2790 | |
| PubChem | 5281107 |
| UNII | 3DIZ9LF5Y9 |
| Properties | |
| C28H44O2 | |
| Molar mass | 412.66 g·mol−1 |
| Pharmacology | |
| H05BX03 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
/////////////
No comments:
Post a Comment