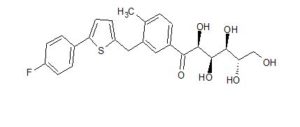
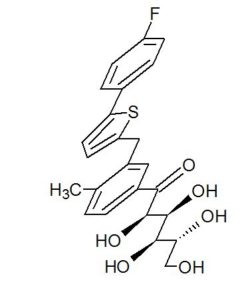
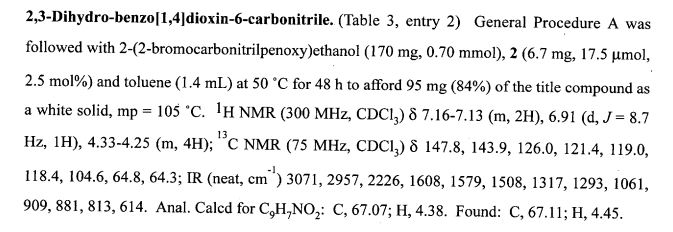
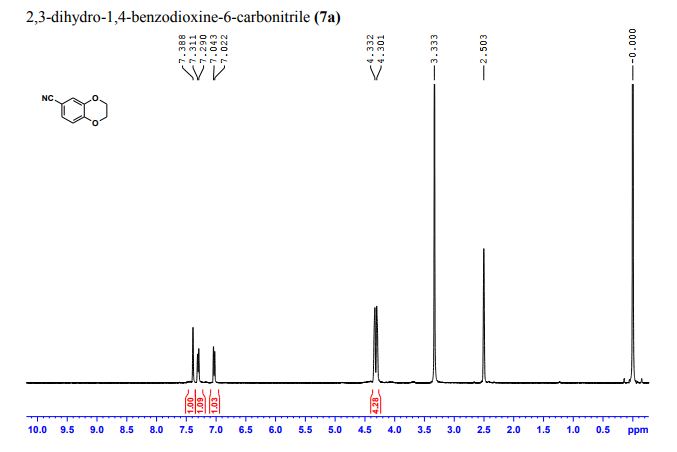
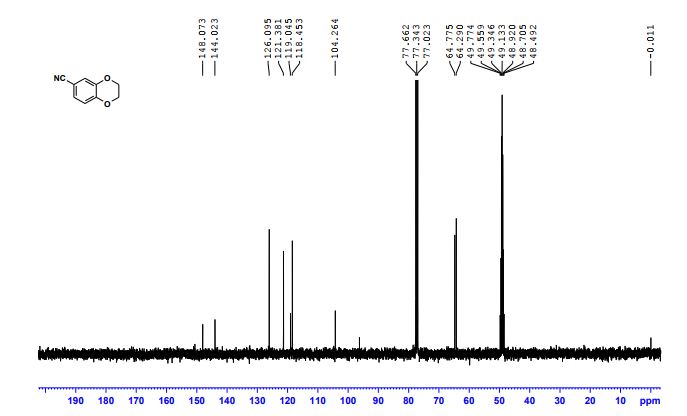
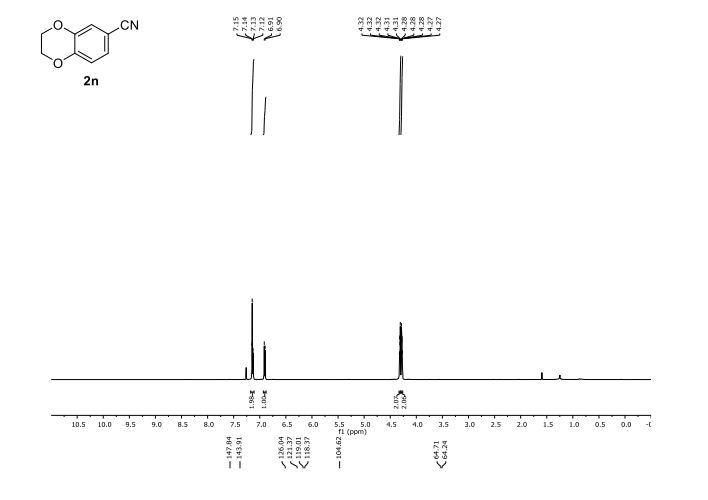
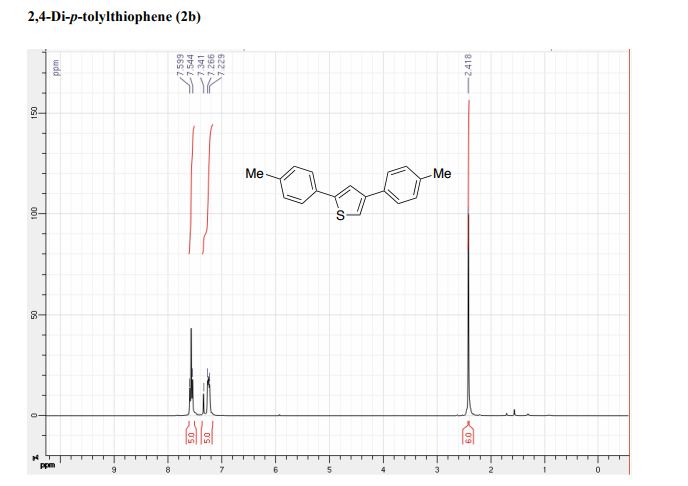
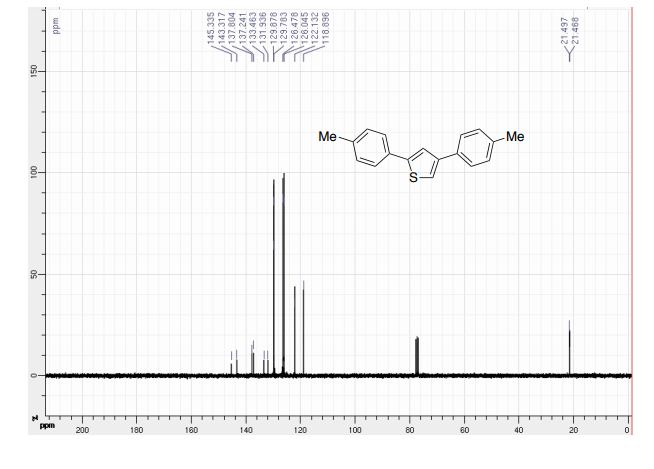
CAS 1672658-93-3
C24 H25 F O6 S, 460.52
D-Glucopyranose, 1-C-[3-[[5-(4-fluorophenyl)-2-thienyl]methyl]-4-methylphenyl]-
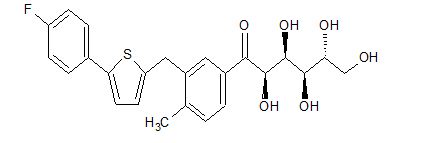
CAS 1809403-04-0
C24 H25 F O6 S, 460.52
D-Glucose, 1-C-[3-[[5-(4-fluorophenyl)-2-thienyl]methyl]-4-methylphenyl]-
WO2017/93949

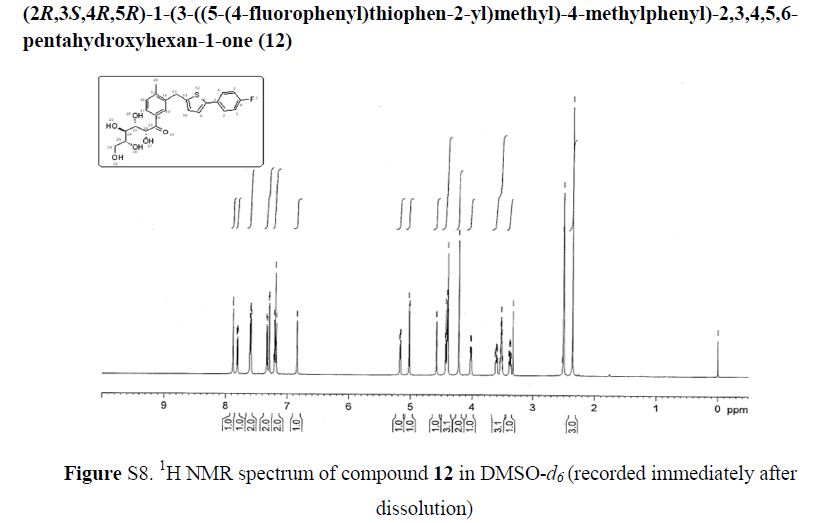
(2R,3S,4R,5R)-1-(3-((5-(4-Fluorophenyl)thiophen-2-yl)methyl)-4-methylphenyl)-2,3,4,5,6-pentahydroxyhexan-1-one 12
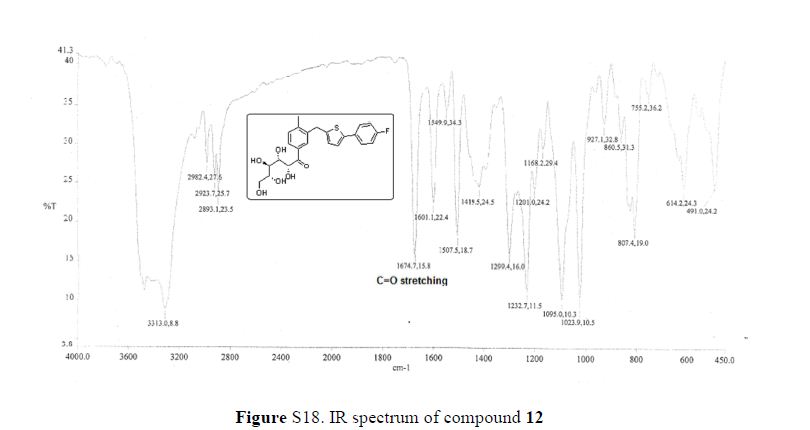
From the FT-IR spectra of 12 contain a signal at 1674 cm–1, this signal is strongly indicative of a carbonyl ketone being present in 12
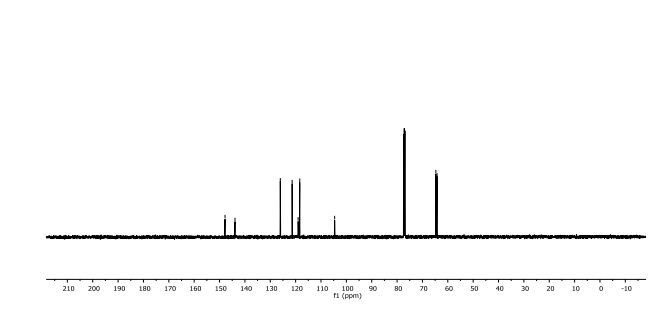
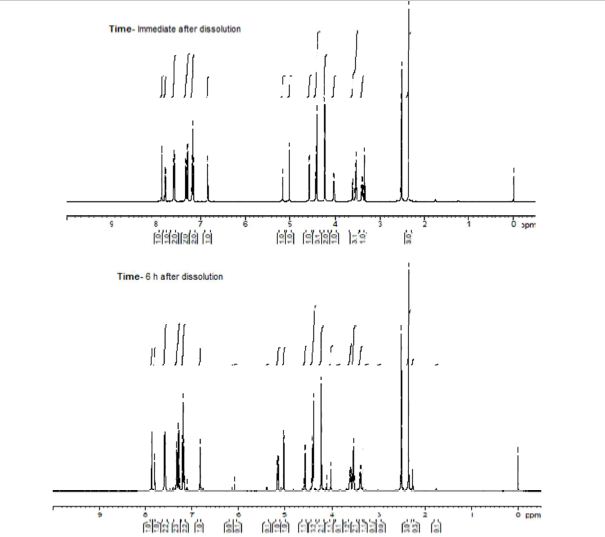
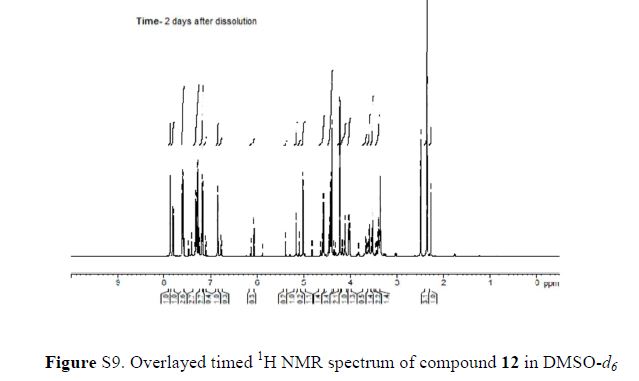
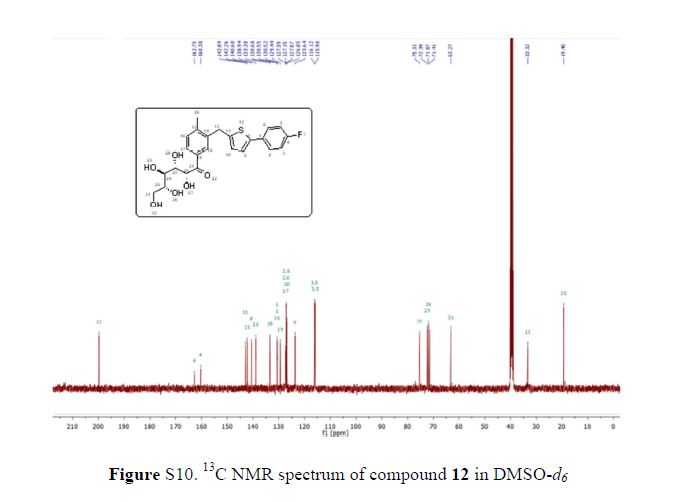
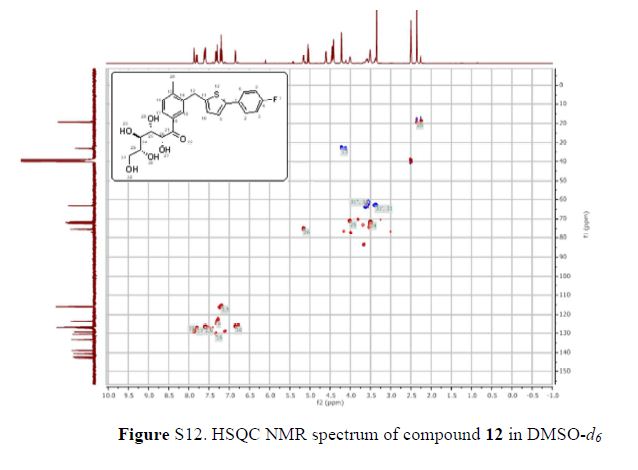
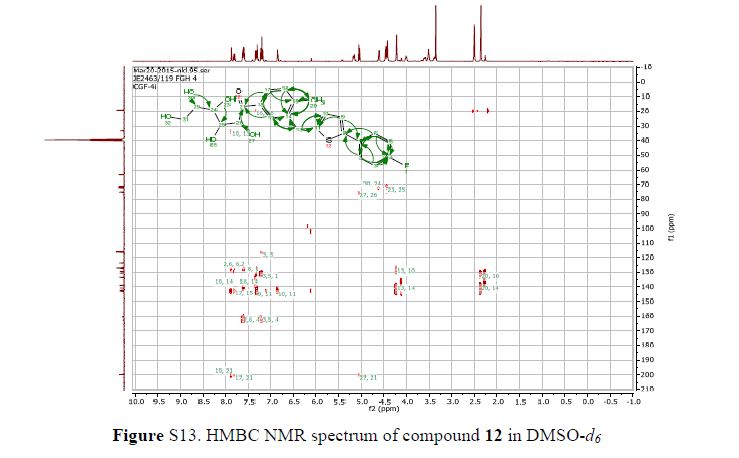
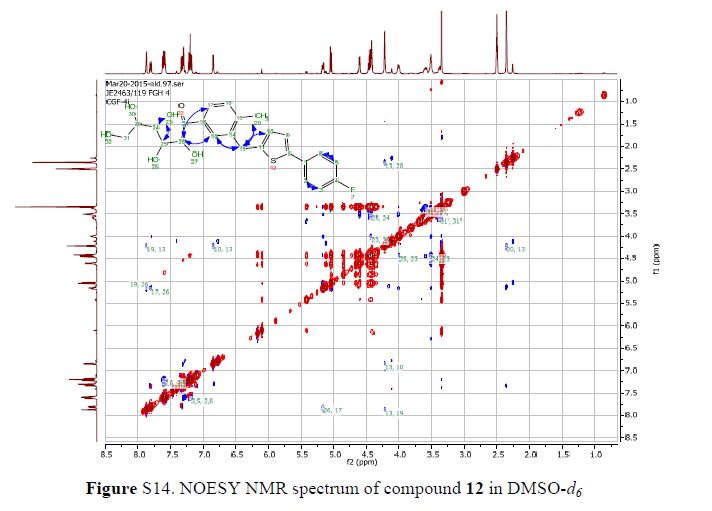
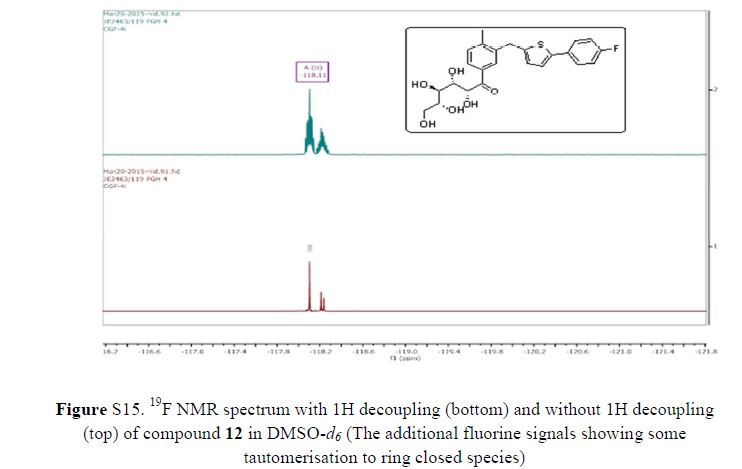
In 13C NMR and HMBC correlations spectra, the chemical shift at 199.75 ppm was observed. Analysis of the NMR data confirmed that the structure of 12 is a ring-opened keto form
Synthesis of (2R,3S,4R,5R)-1-(3-((5-(4-Fluorophenyl)thiophen-2-yl)methyl)-4-methylphenyl)-2,3,4,5,6-pentahydroxyhexan-1-one 12
title compound 12 (84.23% yield) and having 99.4% purity by HPLC;
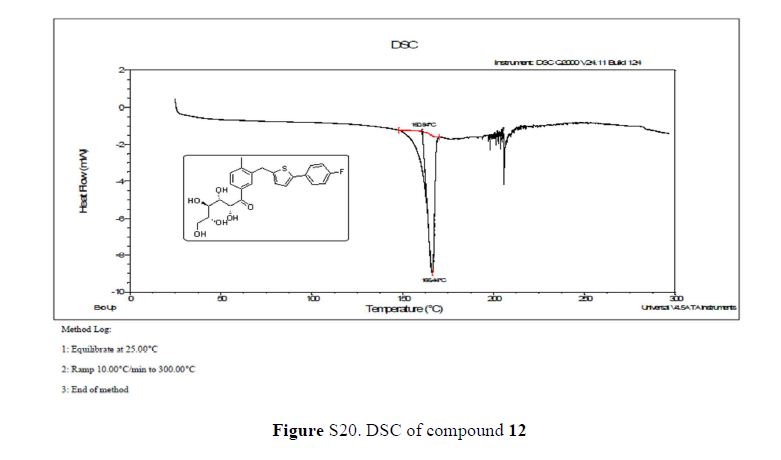
DSC: 160.84–166.44 °C;
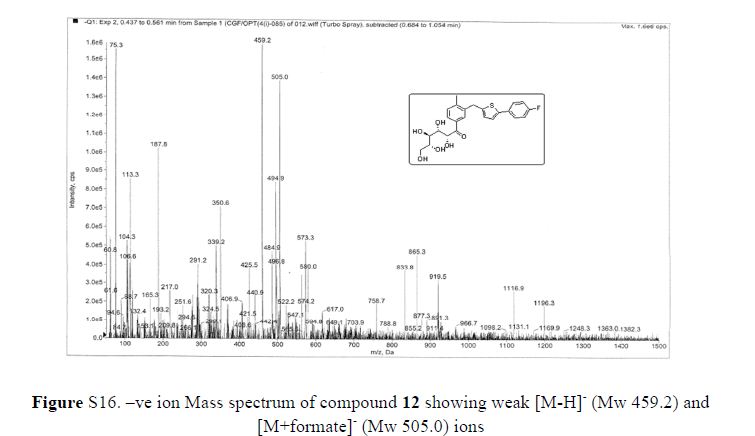
Mass: m/z 459 (M+–H);
IR (KBr, cm–1): 3313, 2982, 1674.7, 1601, 1507.5, 1232.7;
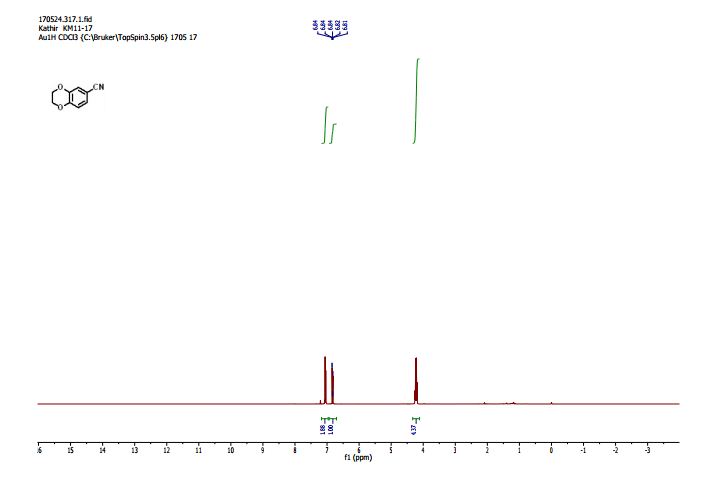
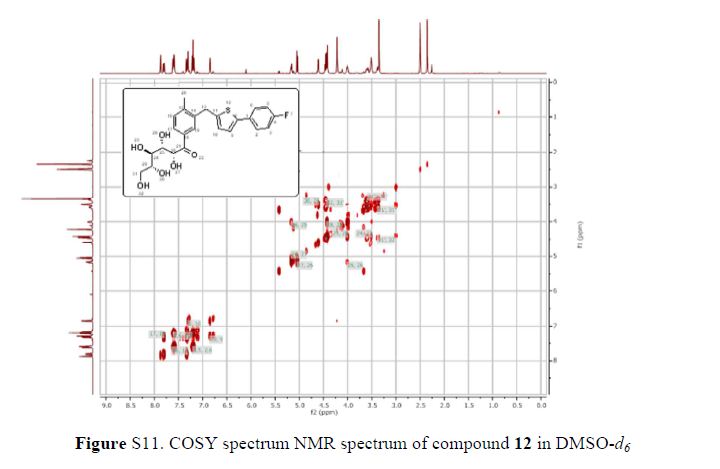
1H NMR (600 MHz, DMSO-d6) δ 7.87 (s, 1H), 7.80 (dd, J = 1.8 Hz, 1H), 7.61–7.58 (m, 2H), 7.33 (d, J = 8.4 Hz, 1H), 7.29 (d, J = 3.6 Hz, 1H), 7.21–7.18 (m, 2H), 6.84 (d, J = 3.6 Hz, 1H), 5.17 (dd, J = 3.6, 3.0 Hz, 1H), 5.02 (d, J = 6.6 Hz, 1H), 4.57 (d, J = 4.8 Hz, 1H), 4.43–4.39 (m, 3H), 4.22 (s, 2H), 4.02–4.01 (m, 1H), 3.53–3.51 (m, 3H), 3.38–3.37 (m, 1H), 2.35 (s, 3H);
13C NMR (101 MHz, DMSO-d6) δ 199.7, 162.6, 160.2, 142.8, 142.1, 140.5, 138.8, 133.3, 130.5, 130.4, 130.4, 129.3, 127.2, 127.0, 127.0, 126.7, 123.5, 116.0, 115.8, 75.2, 72.3, 71.8, 71.3, 63.2, 33.2, 19.2.
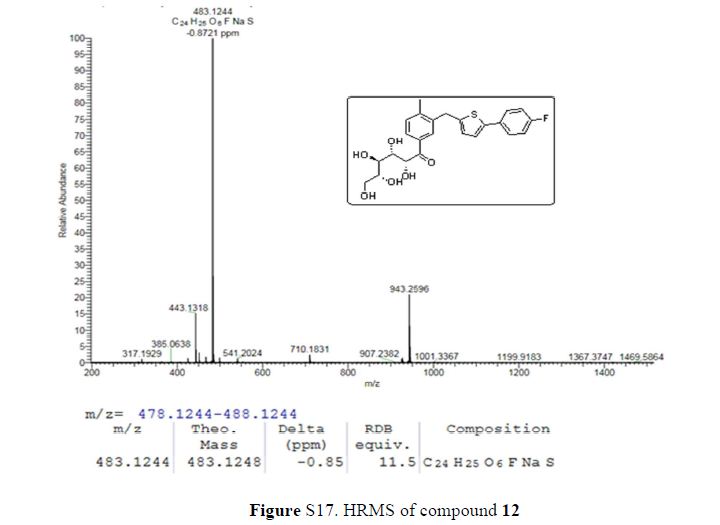
HRMS (ESI): calcd m/zfor [C24H25FO6S + Na]+ = 483.1248, found m/z 483.1244.
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.7b00281
////////////