Green Chem., 2018, 20,973-977
DOI: 10.1039/C7GC03719H, Communication
Fuhong Xiao, Chao Liu, Dahan Wang, Huawen Huang, Guo-Jun Deng
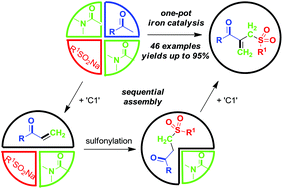
A three starting material four component reaction (3SM-4CR) strategy is described to prepare [small beta]-acyl allylic sulfones from methyl ketones, sodium sulfinates and dimethylacetamide (DMA) in an iron-catalyzed oxidative system.
Concise synthesis of ketoallyl sulfones through an iron-catalyzed sequential four-component assembly
Author affiliations
* Corresponding authors
a Key Laboratory of Environmentally Friendly Chemistry and Application of Ministry of Education, Key Laboratory for Green Organic Synthesis and Application of Hunan Province, College of Chemistry, Xiangtan University, Xiangtan 411105, China
E-mail: fhxiao@xtu.edu.cn,
gjdeng@xtu.edu.cn
Abstract
A three starting material four component reaction (3SM-4CR) strategy is described to prepare β-acyl allylic sulfones from methyl ketones, sodium sulfinates and dimethylacetamide (DMA) in an iron-catalyzed oxidative system. In this process, DMA was used as a dual synthon to provide two carbons. A broad range of functional groups were tolerated in this reaction system.
1-phenyl-2-(tosylmethyl)prop-2-en-1-one (3ab)
43.2 mg, 72% yield).
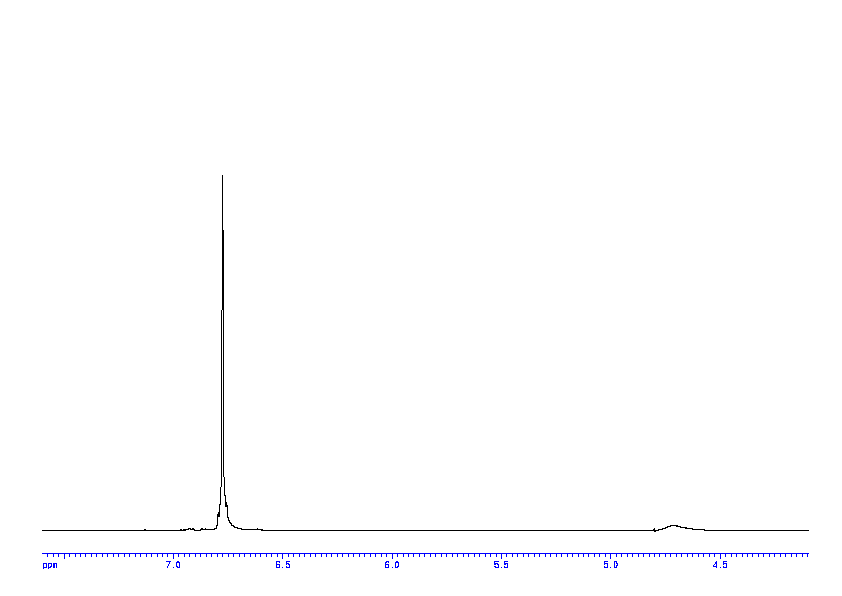
1 H NMR (400 MHz, CDCl3) δ 7.78 (d, J = 8.2 Hz, 2H), 7.68-7.65 (m, 2H), 7.55 (t, J = 7.4 Hz, 1H), 7.43 (t, J = 7.8 Hz, 2H), 7.30 (d, J = 8.3 Hz, 2H), 6.25 (s, 1H), 6.02 (s, 1H), 4.35 (s, 2H), 2.39 (s, 3H).
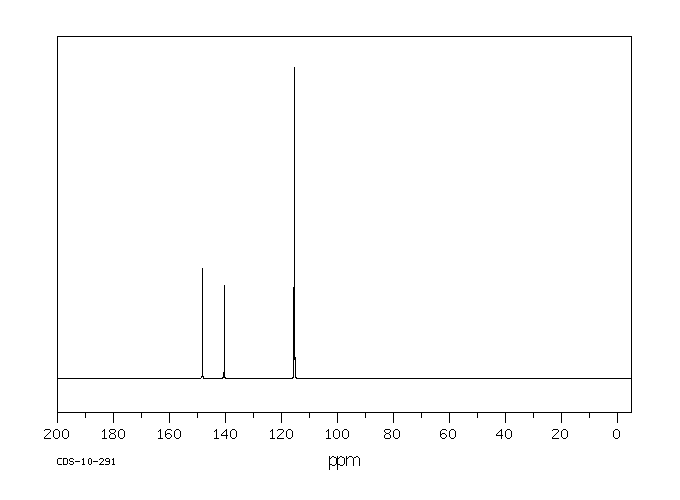
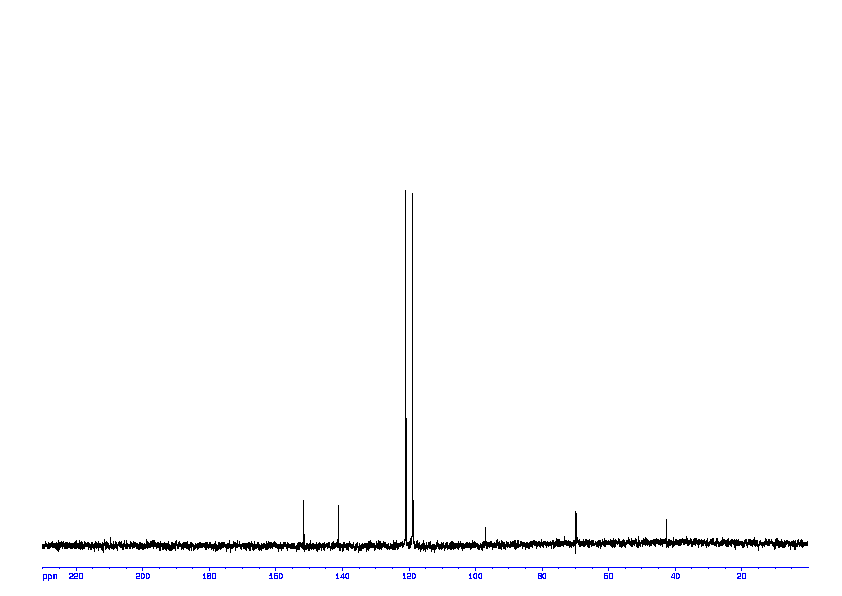
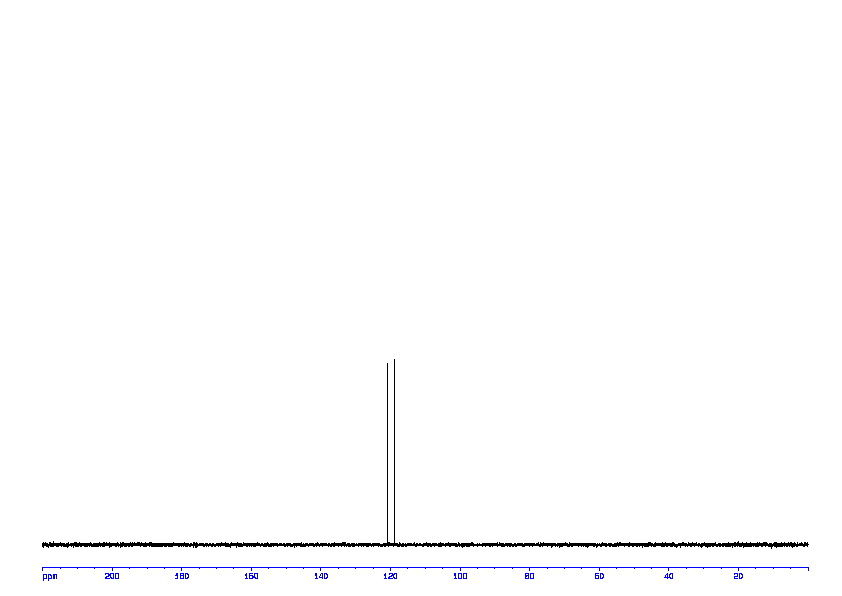
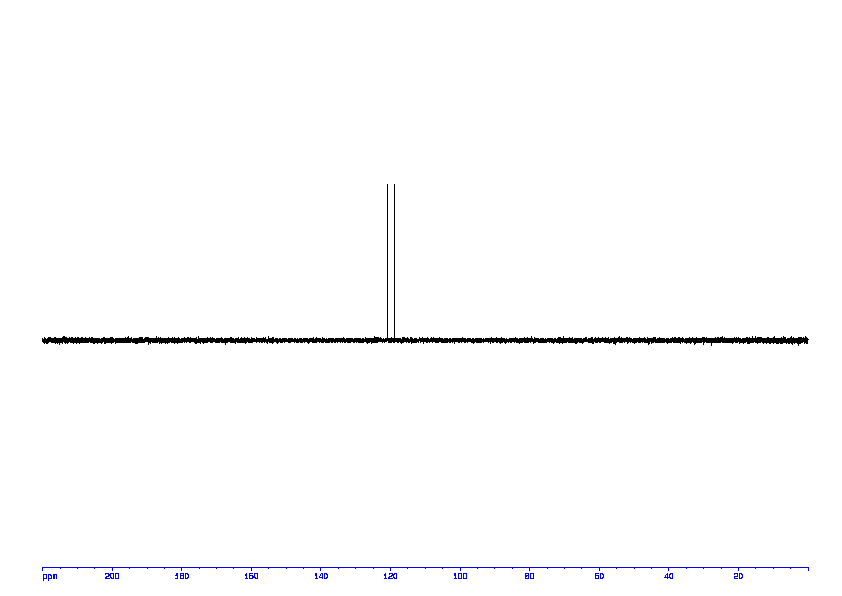
13C NMR (100 MHz, CDCl3) δ 194.7, 144.9, 136.1, 135.8, 135.7, 133.9, 132.6, 129.8, 129.6, 128.3, 128.2, 57.7, 21.6.
HRMS calcd. for: C17H17O3S+ [M+H]+ 301.08929, found 301.08908
1H NMR PREDICT
13C NMR PREDICT ABOVE
////////
Cc1ccc(cc1)S(=O)(=O)CC(=C)C(=O)c2ccccc2






![2D [1H,1H]-TOCSY, 7.4 spectrum for 4-Aminophenol](http://www.bmrb.wisc.edu/ftp/pub/bmrb/metabolomics/entry_directories/bmse000462/nmr/set01/spectra/HH_TOCSY.png)
![2D [1H,13C]-HSQC, 7.4 spectrum for 4-Aminophenol](http://www.bmrb.wisc.edu/ftp/pub/bmrb/metabolomics/entry_directories/bmse000462/nmr/set01/spectra/1H_13C_HSQC.png)
![2D [1H,13C]-HMBC, 7.4 spectrum for 4-Aminophenol](http://www.bmrb.wisc.edu/ftp/pub/bmrb/metabolomics/entry_directories/bmse000462/nmr/set01/spectra/1H_13C_HMBC.png)