Organic Chemists from Industry and academics to Interact on Spectroscopy Techniques for Organic Compounds ie NMR, MASS, IR, UV Etc. Starters, Learners, advanced, all alike, contains content which is basic or advanced, by Dr Anthony Melvin Crasto, Worlddrugtracker, email me ........... amcrasto@gmail.com, call +91 9323115463 India skype amcrasto64
................DR ANTHONY MELVIN CRASTO Ph.D ( ICT, Mumbai) , INDIA 25Yrs Exp. in the feld of Organic Chemistry,Working for GLENMARK GENERICS at Navi Mumbai, INDIA. Serving chemists around the world. Helping them with websites on Chemistry.Million hits on google, world acclamation from industry, academia, drug authorities for websites, blogs and educational contribution
Pages
- Home
- ABOUT ME
- DIMENSIONS IN NMR SPECTROSCOPY
- 13 C NMR
- 1H NMR
- CHEMDOODLE/INTERACTIVE SPECT PREDICT
- Animations
- HELP ME
- Multinuclear NMR Spectroscopy
- Examples of 13C NMR
- Books on NMR spectroscopy
- UV-Visible Spectroscopy
- IR SPECTRA EXAMPLES
- Journals
- Organic spectroscopy site
- Spectroscopy sites
- IR SPECTROSCOPY
- Books-2
- Recommended Web Sites for Spectra and Spectrum-rel...
- DISCLAIMER
- Mössbauer spectroscopy
- FINDING CHEMICAL SPECTRA
- Mass Spectrometry
- NMR Overview
- Characterisation of Organic Compounds
- SDBS Spectral Database System for Organic Compounds
- CHEMICAL SHIFT
- MASS SPECTROSCOPY
- Books-1
- MASSBANK PORTAL
- 11B NMR
Tuesday, 2 April 2019
Wednesday, 27 March 2019
Taselisib

TASELISIB
SYNTHESIS........https://newdrugapprovals.org/2018/06/15/rg-7604taselisib/
CLIP
Manufacture of the PI3K β-Sparing Inhibitor Taselisib. Part 2: Development of a Highly Efficient and Regioselective Late-Stage Process
Frédéric St-Jean*†  , Travis Remarchuk†, Rémy Angelaud*†, Diane E. Carrera†, Danial Beaudry†, Sushant Malhotra†, Andrew McClory†, Archana Kumar†, Gerd Ohlenbusch‡, Andreas M. Schuster‡, and Francis Gosselin†
, Travis Remarchuk†, Rémy Angelaud*†, Diane E. Carrera†, Danial Beaudry†, Sushant Malhotra†, Andrew McClory†, Archana Kumar†, Gerd Ohlenbusch‡, Andreas M. Schuster‡, and Francis Gosselin†
 , Travis Remarchuk†, Rémy Angelaud*†, Diane E. Carrera†, Danial Beaudry†, Sushant Malhotra†, Andrew McClory†, Archana Kumar†, Gerd Ohlenbusch‡, Andreas M. Schuster‡, and Francis Gosselin†
, Travis Remarchuk†, Rémy Angelaud*†, Diane E. Carrera†, Danial Beaudry†, Sushant Malhotra†, Andrew McClory†, Archana Kumar†, Gerd Ohlenbusch‡, Andreas M. Schuster‡, and Francis Gosselin†
† Department of Small Molecule Process Chemistry, Genentech Inc., 1 DNA Way, South San Francisco, California 94080, United States
‡ Small Molecules Technical Development PTDC-C, F. Hoffmann-La Roche Ltd., Grenzacherstrasse 124, 4070 Basel, Switzerland
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.9b00050
*E-mail: stjean.frederic@gene.com. (F.St.-J.), *E-mail: angelaud.remy@gene.com. (R.A.)
A highly efficient and regioselective manufacturing route for the phosphoinositide 3-kinase β-sparing inhibitor taselisib was developed. Highlights of the synthesis include: (1) magnesium-mediated formation of a challenging cyclic amidine; (2) regioselective imidazole construction via alkylation/condensation with bromopyruvic acid; and (3) triazole formation with 2-isopropyl acetamidrazone to generate the key bromobenzoxazepine core intermediate. Subsequent highly efficient one-pot palladium-catalyzed Miyaura borylation/Suzuki cross-coupling/saponification, followed by a 1,1′-carbonyldiimidazole-mediated coupling with ammonia, led to the pentacyclic taselisib. This new synthetic approach provides a more efficient route to taselisib with a significant decrease in process mass intensity compared to the previous early development routes to the bromobenzoxazepine core. Finally, implementation of a controlled crystallization provided the active pharmaceutical ingredient with the desired polymorphic form.
Taselisib was obtained in 90% yield (16.2 kg) as a white to off-white solid (>99.9 wt %; >99.9 A%) as Form B crystal.
mp (DSC): 257–258 °C.
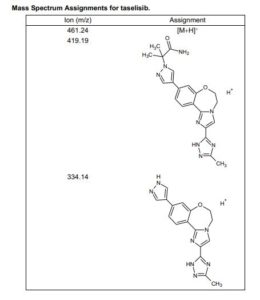
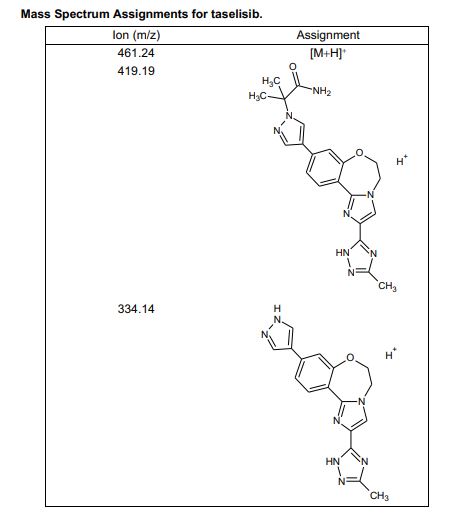
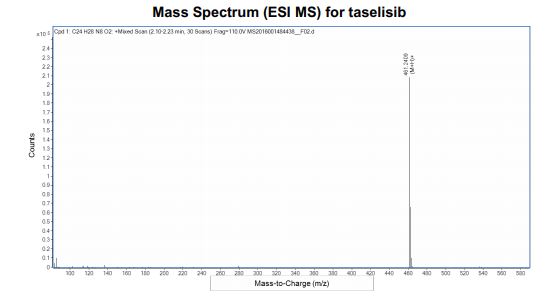
HRMS (ESI-CID) m/z Calcd for [M + H]+ C24H29N8O2: 461.2408; found: 461.2409.
1H NMR (600 MHz, DMSO-d6) δ ppm 8.41 (s, 1H), 8.37 (d, J = 8.4, 1H), 8.02 (s, 1H), 7.88 (m, 1H), 7.45 (dd, J = 8.4, 1.7 Hz, 1H), 7.36 (d, J = 1.7 Hz, 1H), 7.20 (br s, 1H), 6.84 (br s, 1 H), 5.83 (sept, J = 6.6 Hz, 1H), 4.53–4.51 (m, 2H), 4.52 (m, 2H), 2.26 (s, 3H), 1.75 (s, 6H), 1.47 (d, J = 6.7 Hz, 6H).
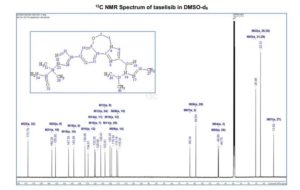
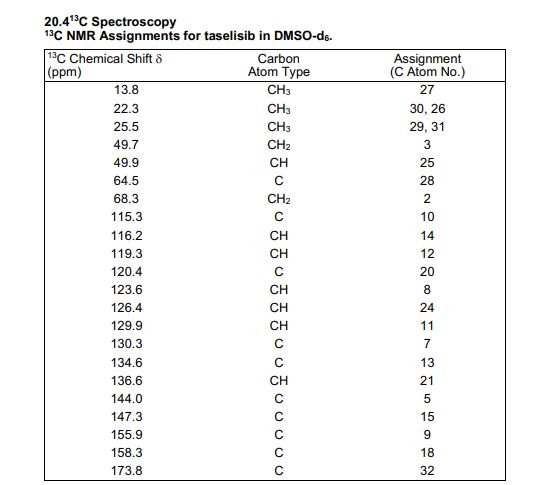
13C NMR (DMSO-d6, 151 MHz) δ = 173.8, 158.3, 155.9, 147.3, 144.0, 136.6, 134.6, 130.3, 129.9, 126.4, 123.6, 120.4, 119.3, 116.2, 115.3, 68.3, 64.5, 49.9, 49.7, 25.5, 25.5, 22.3, 22.3, 13.8.
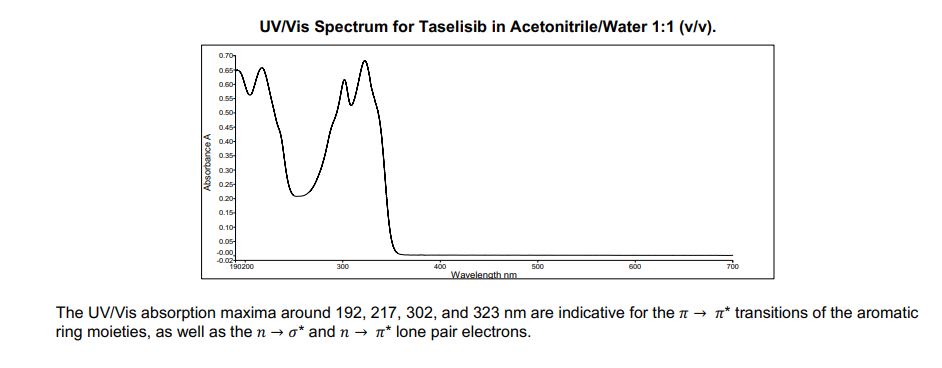
Ultraviolet/Visible Spectroscopy
Apparatus: Perkin Elmer, UV-6190, Lambda 25
Solvent: Acetonitrile/Water 1:1 (v/v)
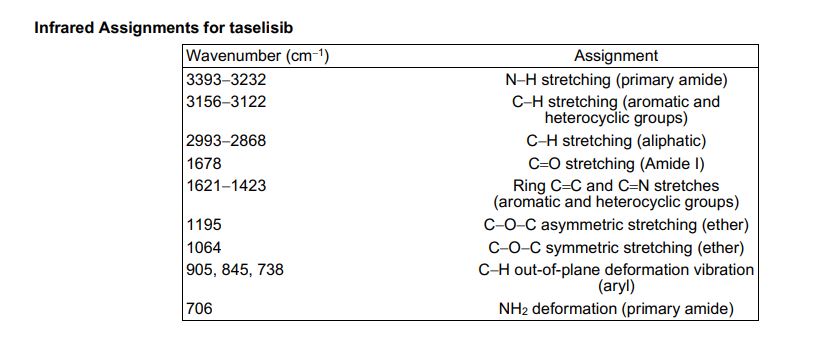
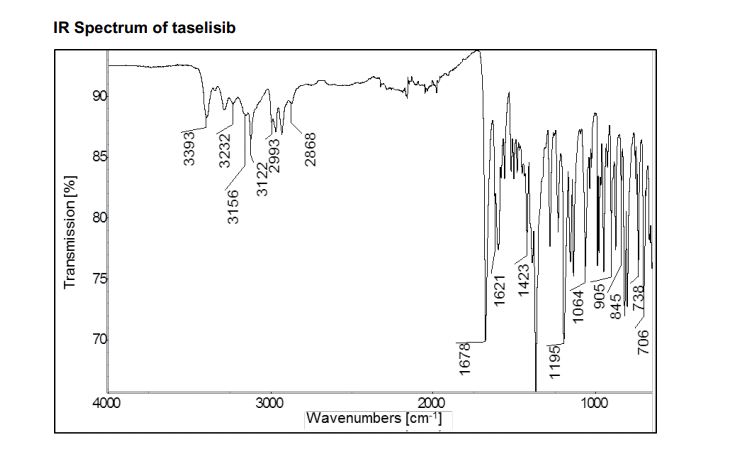
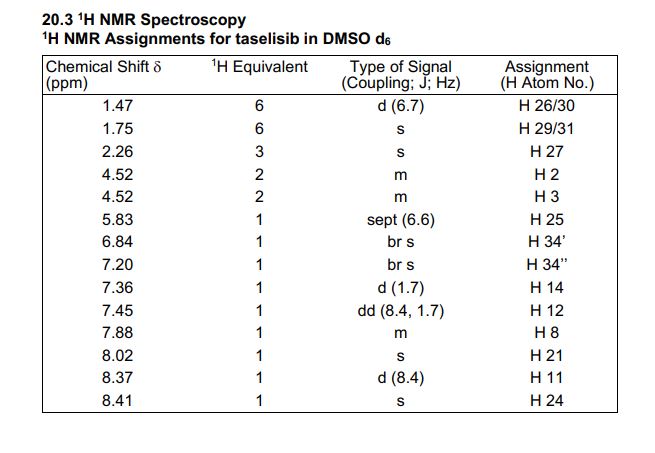
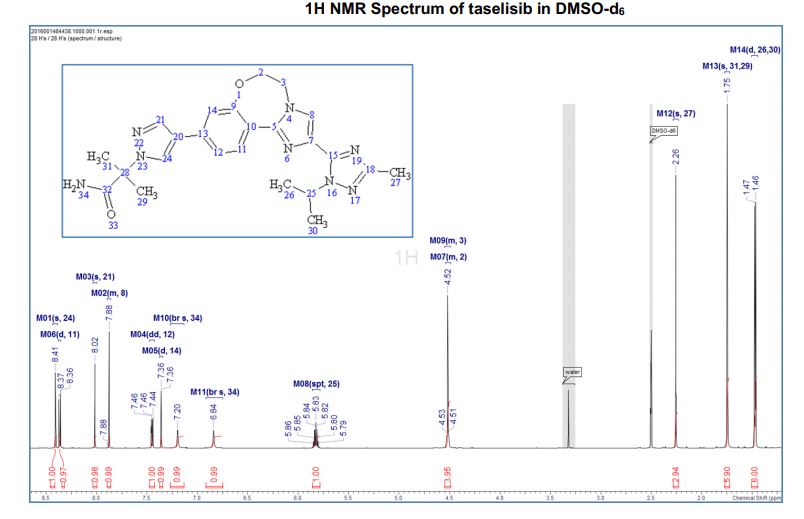

////////////////
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This is a compilation for educational purposes only. P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
READ
ANTHONY MELVIN CRASTO
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
Join me on google plus  Googleplus
Googleplus
 amcrasto@gmail.com
amcrasto@gmail.com
CALL +919323115463 INDIA
//////////////
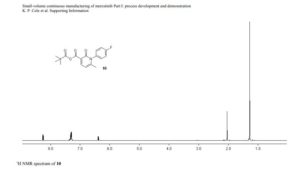
1-(4-fluorophenyl)-6-methyl-2-oxo-1,2-dihydropyridine-3-carboxylic pivalic anhydride
1-(4-fluorophenyl)-6-methyl-2-oxo-1,2-dihydropyridine-3-carboxylic pivalic anhydride (10): tan solid,
mp (DSC): onset = 172.4 °C, peak temperature = 173.0 °C;
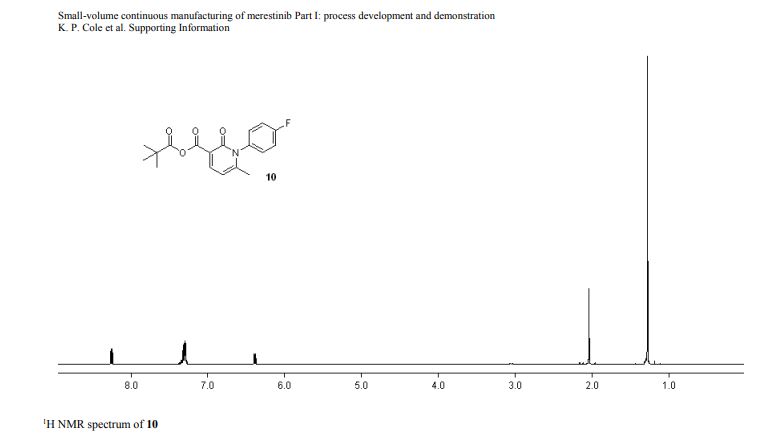
1H NMR (400 MHz, CD3CN) δ 8.23 (d, J = 7.6 Hz, 1 H), 7.35–7.25 (m, 4 H), 6.36 (d, J = 8.0 Hz, 1 H), 2.02 (s, 3 H), 1.25 (s, 9 H);
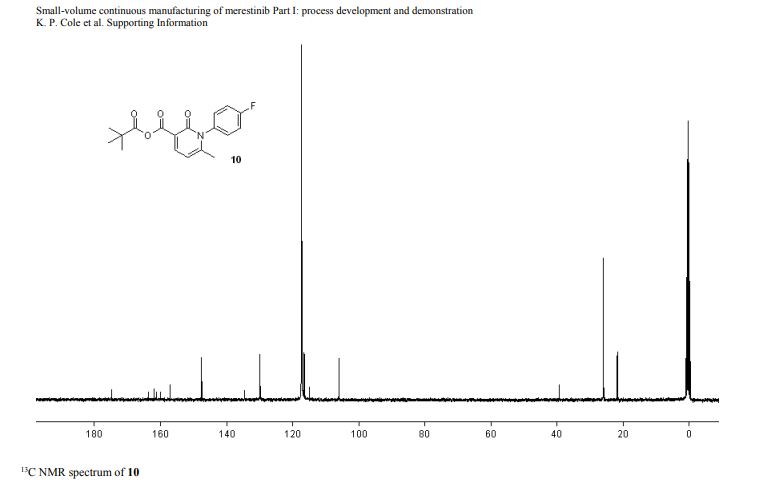
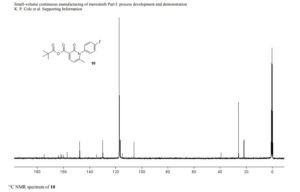
13C NMR (100 MHz, CD3CN) 175.9 (1 C), 163.6 (d, 1 C, JCF = 245.4 Hz), 162.9 (1 C ), 161.1 (1 C), 158.2 (1 C), 148.6 (1 C), 135.7 (1 C), 131.0 (d, JCF = 8.7 Hz, 2 C), 117.7 (d, JCF = 23.2 Hz, 2 C), 116.0 (1 C), 107.1 (1 C), 40.4 (1 C), 27.0 (3 C), 22.8 (1 C);
19F NMR (376 MHz, CD3CN) δ -114.2 (m, 1 F);
IR (ATR, cm-1 ) 2966 (w), 2939 (w), 2876 (w), 1789 (vs), 1697 (s), 1682 (vs), 1554 (s), 1509 (vs);
HRMS calcd for C18H18O4NFNa (M+Na+ ) = 354.1112; found 354.1113, δ = 0.3 ppm.
//////////////https://pubs.acs.org/doi/suppl/10.1021/acs.oprd.8b00441
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This is a compilation for educational purposes only. P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
READ
ANTHONY MELVIN CRASTO
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
Join me on google plus  Googleplus
Googleplus
 amcrasto@gmail.com
amcrasto@gmail.com
CALL +919323115463 INDIA
//////////////
Thursday, 31 January 2019
Telescoped Sequence of Exothermic and Endothermic Reactions in Multistep Flow Synthesis
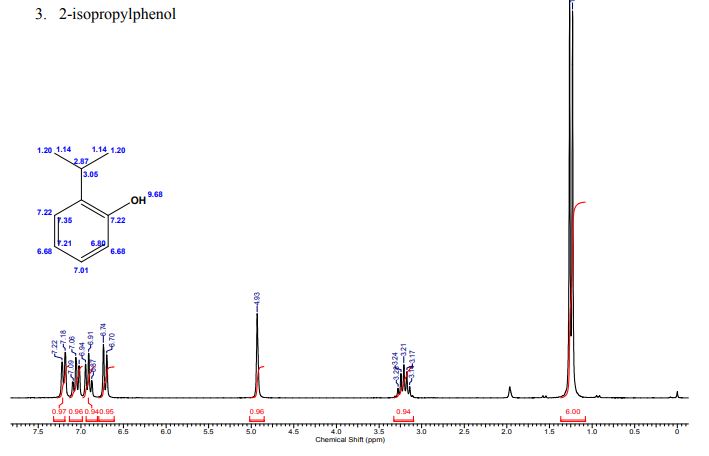
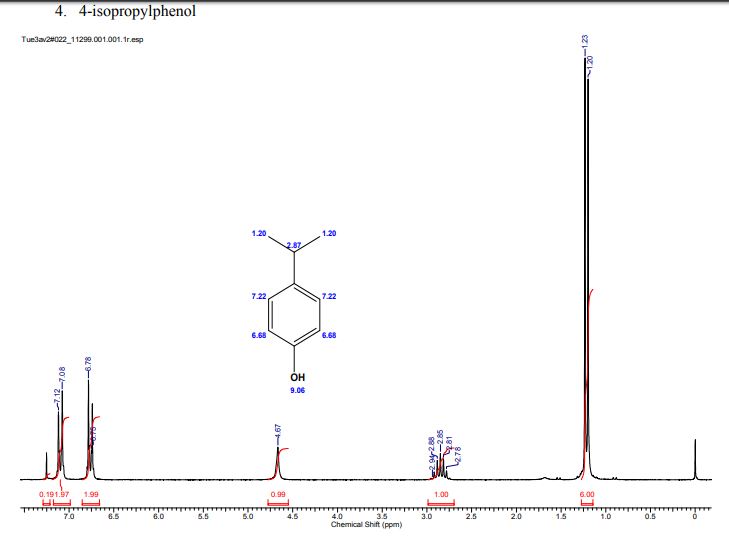
A multistep sequential flow synthesis of isopropyl phenol is demonstrated, involving 4-step exothermic, endothermic, and temperature sensitive reactions such as nitration, reduction, diazotization, and high temperature hydrolysis. Nitration of cumene with fuming nitric acid produces 2- and 4-nitrocumene which are converted into respective cumidines by the hydrogenation using Pd/Ni catalyst in H-cube with gravity separation. Hydrolysis of in situ generated diazonium salts in the boiling acidic conditions is carried out using integration of flow and microwave-assisted synthesis. 58% of 4-isopropyl phenol was obtained. The sequential flow synthesis can be applied to synthesize other organic compounds involving this specific sequence of reactions.
Telescoped Sequence of Exothermic and Endothermic Reactions in Multistep Flow Synthesis
Chemical Engineering & Process Development Division, CSIR-National Chemical Laboratory, Dr. Homi Bhabha Road, Pune 411008, India
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.8b00008
*Phone: +91-20-25902153, E-mail: aa.kulkarni@ncl.res.in.

Dr.Amol A. Kulkarni
Chemical Engineering & Process Development
CSIR-National Chemical Laboratory
Chemical Engineering & Process Development
CSIR-National Chemical Laboratory

Dr. Amol A. Kulkarni is a Scientist in the Chemical Engineering Division at the National Chemical Laboratory. He did his B. Chem. Eng. (1998), M. Chem. Eng (2000) and Ph.D. in chemical engineering (2003) all from the University Dept. of Chem. Technology (UDCT, Mumbai). In 2004 he worked at the Max Planck Institute-Magdeburg (Germany) as a Alexander von Humboldt Research Fellow. At NCL he is driving a research program on the design of microreactors and exploring their applications for continuous syntheses including of nanoparticles. He has been awarded with the Max-Planck-Visiting Fellowship from the Max-Planck-Society, Munich for 2008-2011. His research areas include: (i) design and applications of microreactors, (ii) design of multiphase reactors, (iii) experimental and computational fluid dynamics, and (iv) nonlinear dynamics of coupled systems. He is an active member of Initiative for Research and Innovation in Science (IRIS) supported by Intel’s Education Initiative to organize National Science Fair and popularize science in India.

Yachita Sharma
Location Pune, India
Yachita Sharma is a PhD student at CSIR-National Chemical Laboratory, Pune (India). She received her MSc in Applied Organic Chemistry in 2010. Her work focuses on exploring the continuous flow synthesis involving exothermic reactions and their integration.

Arun Nikam
Location, Pune, India
Email: arun11nikam@gmail.com
Arun was born and raised in Koregaon, Maharashtra, India. He completed his bachelors and masters in chemical sciences from Shivaji Unversity, Kolhapur, India. Currently, He is pursuing his Ph. D. under the supervision of Dr. Amol A. Kulkarni and Dr. B. L. V. Prasad. His work mainly focuses on flow synthesis of nanoparticles, drug formulation, and polymers. He develops new synthesis procedures and translates into flow chemistry to increase productivity with excellent control over the quality of the product. He is also exploring the application of nanoparticles in catalysis, electronics and pharmaceutical fields. He specializes in microwave-assisted continuous flow synthesis of nanomaterial and organic intermediate. Apart from his research, he actively pursues Yoga and spirituality. He also likes to play volleyball and has competed in inter CSIR tournaments.
Arun was born and raised in Koregaon, Maharashtra, India. He completed his bachelors and masters in chemical sciences from Shivaji Unversity, Kolhapur, India. Currently, He is pursuing his Ph. D. under the supervision of Dr. Amol A. Kulkarni and Dr. B. L. V. Prasad. His work mainly focuses on flow synthesis of nanoparticles, drug formulation, and polymers. He develops new synthesis procedures and translates into flow chemistry to increase productivity with excellent control over the quality of the product. He is also exploring the application of nanoparticles in catalysis, electronics and pharmaceutical fields. He specializes in microwave-assisted continuous flow synthesis of nanomaterial and organic intermediate. Apart from his research, he actively pursues Yoga and spirituality. He also likes to play volleyball and has competed in inter CSIR tournaments.
/////////isopropyl phenol, flow chem,
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This is a compilation for educational purposes only. P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
READ
ANTHONY MELVIN CRASTO
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
Join me on google plus  Googleplus
Googleplus
 amcrasto@gmail.com
amcrasto@gmail.com
CALL +919323115463 INDIA
//////////////
Subscribe to:
Posts (Atom)