A catalyst-free 1,3-dipolar cycloaddition of C,N-cyclic azomethine imines and 3-nitroindoles: an easy access to five-ring-fused tetrahydroisoquinolines
A catalyst-free 1,3-dipolar cycloaddition of C,N-cyclic azomethine imines and 3-nitroindoles: an easy access to five-ring-fused tetrahydroisoquinolines
*
Corresponding authors
a
School of Life Sciences, Institute of Biochemistry and Molecular Biology, Lanzhou University, Lanzhou 730000, P. R. China
E-mail: wangrui@lzu.edu.cn
E-mail: wangrui@lzu.edu.cn
b
State Key Laboratory of Chiroscience, Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Kowloon, P. R. China
E-mail: bcrwang@polyu.edu.hk
E-mail: bcrwang@polyu.edu.hk
Green Chem., 2016, Advance Article
DOI: 10.1039/C6GC02517J
We have reported herein a catalyst-free 1,3-dipolar cycloaddition of C,N-cyclic azomethine imines and 3-nitroindoles by which a series of five-ring-fused tetrahydroisoquinolines featuring an indoline scaffold were obtained as single diastereomers in moderate to high yields without any additives under mild conditions. Moreover, the current method provides a novel and convenient approach for the efficient incorporation of two biologically important scaffolds (tetrahydroisoquinoline and indoline).
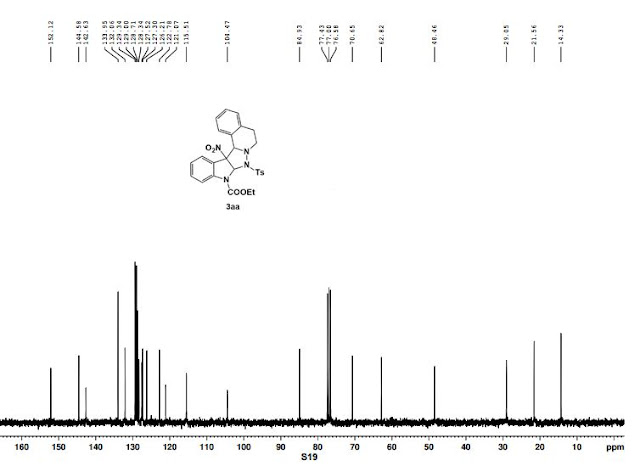
ethyl 13b-nitro-8-tosyl-8,8a,13b,13c-tetrahydro-5H-indolo[2',3':3,4]pyrazolo[5,1- a]isoquinoline-9(6H)-carboxylate
ethyl 13b-nitro-8-tosyl-8,8a,13b,13c-tetrahydro-5H-indolo[2',3':3,4]pyrazolo[5,1- a]isoquinoline-9(6H)-carboxylate:
White solid, m.p. 153 – 154 oC; 94% yield; 1H NMR (300 MHz, CDCl3) δ 7.86 (d, J = 8.2 Hz, 2H), 7.78 (d, J = 7.9 Hz, 1H), 7.30 – 7.13 (m, 5H), 7.1 (s, 1H), 7.05 – 6.94 (m, 1H), 6.94 – 6.87 (m, 1H), 6.59 (t, J = 7.6 Hz, 3H), 6.28 (d, J = 7.6 Hz, 1H), 4.78 (s, 1H), 4.37 (q, J = 7.1 Hz, 2H), 2.80 – 2.58 (m, 2H), 2.33 (s, 3H), 2.31 – 2.11 (m, 2H), 1.41 (t, J = 7.1 Hz, 3H) ppm;
13C NMR (75 MHz, CDCl3) δ 152.1, 144.6, 142.6, 134.0, 132.1, 129.3, 129.0, 128.7, 128.3, 127.5, 127.3, 126.2, 122.8, 121.1, 115.5, 104.5, 84.9, 70.7, 62.8, 48.5, 29.1, 21. 6, 14.3 ppm;
HRMS (ESI): C27H26N4NaO6S [M + Na]+ calcd: 557.1465, found: 557.1476.
////////////////


No comments:
Post a Comment