1H NMR PREDICT
13C NMR PREDICT
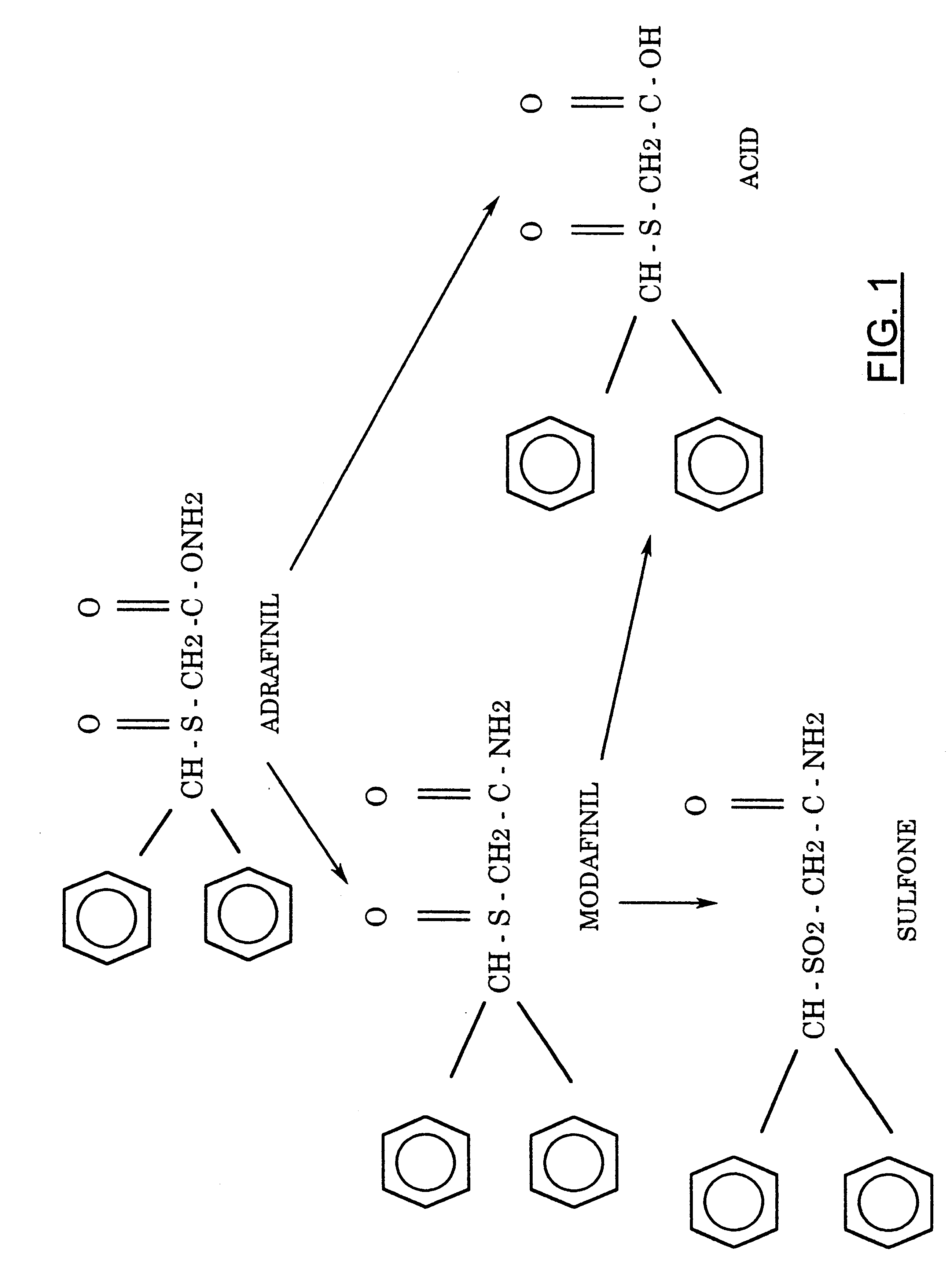
ADRAFINIL
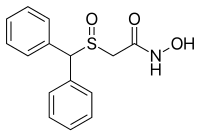
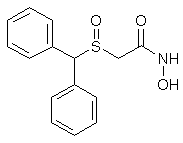
2-((diphenylmethyl)sulfinyl)-acetohydroxamicaci;2-((diphenylmethyl)sulfinyl)-n-hydroxy-acetamid;2-((diphenylmethyl)sulfinyl)-n-hydroxyacetamide;2-(benzhydrylsulfinyl)acetohydroxamicacid;ADRAFINIL;2-[(DIPHENYLMETHYL)SULFINYL]ACETOHYDROXAMIC ACID;CRL 40028;OLMIFON
- CAS 63547-13-7
- MF:C15H15NO3S
- MW:289.35
- EINECS:264-303-1
WATCH THIS POST AS DETAILS LIKE SYNTHESIS ARE UPDATED.............
Adrafinil is touted mainly for its stimulant properties and ability to provide alertness and wakefulness.
- Stay up late/stay awake during normal sleeping hours: Adrafinil may be helpful for night workers who need a kick-start adapting their body’s natural circadian rhythm of wakefulness in the daytime and sleepiness in the evening to their job needs. This can also make it helpful for periodic late-night study sessions. Adrafinil is best taken in the afternoon or evening for nighttime wakefulness.
- Boost energy, alertness, and focus during the day time: Adrafinil can also be used as an energy-boost during waking hours.
- CONTACT SKYPE CATHERINESSPC WICKR
Adrafinil (
INN) (brand name
Olmifon)
[2] is a discontinued
wakefulness-promoting agent (or
eugeroic) that was formerly used in
France to promote
vigilance (alertness),
attention,
wakefulness,
mood, and other parameters, particularly in the elderly.
[3][4] It was also used
off-label by individuals who wished to avoid
fatigue, such as
night workers or others who needed to stay awake and alert for long periods of time. Additionally, "adrafinil is known to a larger nonscientific audience, where it is considered to be a nootropic agent."
[3] Adrafinil is a
prodrug; it is primarily
metabolized in vivo to
modafinil, resulting in very similar
pharmacological effects.
[3] Unlike modafinil, however, it takes time for the
metabolite to accumulate to active levels in the bloodstream. Effects usually are apparent within 45–60 minutes when taken orally on an empty
stomach. Adrafinil was marketed in France under the trade name
Olmifon[2] until September 2011 when it was voluntarily discontinued.
[4]
Pharmacology
Pharmacodynamics
Because
α1-adrenergic receptor antagonists were found to block effects of adrafinil and modafinil in animals, "most investigators assume[d] that adrafinil and modafinil both serve as α
1-adrenergic receptor agonists."
[3] However, adrafinil and modafinil have not been found to bind to the α
1-adrenergic receptor and they lack
peripheral sympathomimetic side effects associated with activation of this receptor;
[5] hence, the evidence in support of this hypothesis is weak, and other mechanisms are probable.
[3] Modafinil was subsequently screened at a variety of targets in 2009 and was found to act as a weak, atypical
blocker of the
dopamine transporter(and hence as a
dopamine reuptake inhibitor), and this action may explain some or all of its pharmacological effects.
[6][7][8] Relative to adrafinil, modafinil possesses greater specificity in its action, lacking or having a reduced incidence of many of the common side effects of the former (including
stomach pain,
skin irritation,
anxiety, and elevated
liver enzymes with prolonged use).
[9][10][11] There is a
case report of two patients that adrafinil may increase interest in sex.
[3] A
case report of adrafinil-induced
orofacial dyskinesia exists.
[12][13] Reports of this side effect also exist for modafinil.
[12]
Pharmacokinetics
In addition to modafinil, adrafinil also produces
modafinil acid (CRL-40467) and
modafinil sulfone (CRL-41056) as metabolites, which form from metabolic modification of modafinil.
History
Adrafinil was discovered in 1974 by two chemists working for the French pharmaceutical company
Laboratoires Lafon who were screening compounds in search of
analgesics.
[14] Pharmacological studies of adrafinil instead revealed
psychostimulant-like effects such as
hyperactivity and
wakefulness in animals.
[14] The substance was first tested in humans, specifically for the treatment of narcolepsy, in 1977–1978.
[14] Introduced by Lafon (now Cephalon), it reached the market in France in 1984,
[4] and for the treatment of narcolepsy in 1985.
[14][15] In 1976, two years after the discovery of adrafinil, modafinil, its
active metabolite, was discovered.
[14] Modafinil appeared to be more potent than adrafinil in animal studies, and was selected for further clinical development, with both adrafinil and modafinil eventually reaching the market.
[14] Modafinil was first approved in France in 1994, and then in the United States in 1998.
[15] Lafon was acquired by
Cephalon in 2001.
[16] As of September 2011, Cephalon has discontinued
Olmifon, its adrafinil product, while modafinil continues to be marketed.
[4]
Society and culture
Regulation
Athletic doping
New Zealand
In 2005 a Medical Classification Committee in
New Zealand recommended to MEDSAFE NZ that adrafinil be classified as a
prescription medicine due to risks of it being used as a
party drug. At that time adrafinil was not scheduled in New Zealand.
[18]
Research
/////////////
SYNTHESIS
Adrafinil (CAS NO.63547-13-7) was discovered in the late 1970s by scientists working with the French pharmaceutical company Group Lafon. First offered in France in 1986 as an experimental treatment for narcolepsy, Lafon later developed modafinil, the primary metabolite of adrafinil. Modafinil possesses greater selective alpha-1 adrenergic activity than adrafinil without the side effects of stomach pain, skin irritations, feelings of tension, and increases in liver enzyme levels.
It is important to monitor the liver of an individual using adrafinil. It can cause liver damage in some instances.
The Adrafinil with CAS registry number of 63547-13-7 is also known as 2-[(Diphenylmethyl)sulfinyl]-N-hydroxyacetamide. The IUPAC name is 2-Benzhydrylsulfinyl-N-hydroxyacetamide. It belongs to product categories of Aromatics Compounds; Aromatics; Intermediates & Fine Chemicals; Pharmaceuticals; Sulfur & Selenium Compounds. This chemical is a light pink solid and its EINECS registry number is 264-303-1. In addition, the formula is C15H15NO3S and the molecular weight is 289.35. This chemical is harmful if swallowed.
Physical properties about Adrafinil are: (1)ACD/LogP: 1.596; (2)ACD/LogD (pH 5.5): 1.60; (3)ACD/LogD (pH 7.4): 1.53; (4)ACD/BCF (pH 5.5): 9.60; (5)ACD/BCF (pH 7.4): 8.34; (6)ACD/KOC (pH 5.5): 175.52; (7)ACD/KOC (pH 7.4): 152.63; (8)#H bond acceptors: 4; (9)#H bond donors: 2; (10)#Freely Rotating Bonds: 6; (11)Index of Refraction: 1.653; (12)Molar Refractivity: 78.858 cm3; (13)Molar Volume: 215.542 cm3; (14)Polarizability: 31.262 10-24cm3; (15)Surface Tension: 67.25 dyne/cm; (16)Density: 1.342 g/cm3
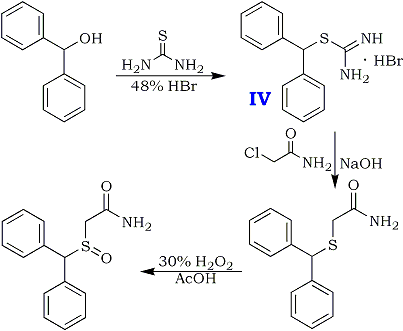
Preparation of Adrafinil: it is prepared by reaction of diphenyl methyl bromide with thiourea. This reaction needs reagent NaOH. After reacting with chloroacetic acid, hydrochloric acid amine and hydrogen peroxide, the product is obtained. The yield is about 73%.
Uses of Adrafinil: it is used as non-amphetamine-type psychostimulant and can wake up and raise awareness. For the elderly arousal disorder and depressive symptoms in symptomatic treatment.
Benzhydrylsulphinyl-acetohydroxamic Acid (Adrafinil)1
Diphenylmethanethiol
15.2 g (0.2 mol) of thiourea and 150 ml of demineralized water are introduced into a 500 ml three-neck flask equipped with a central mechanical stirrer, and with a dropping funnel and a condenser on the (respective) side-necks.The temperature of the reaction mixture is brought to 50°and 49.4g (0.2 mol) of bromodiphenyl- methane are added all at once whilst continuing the heating. After refluxing for about 5 minutes, the solution, which has become limpid, is cooled to 20°C and 200 ml of 2.5 N NaOH are then added dropwise whilst maintaining the said temperature. The temperature is then again kept at the reflex for 30 minutes after which, when the mixture has returned to ordinary temperature (15-25°C), the aqueous solution is acidified with 45 ml of concentrated hydrochloric acid. The supernatant oil is extracted with 250 ml of diethyl ether and the organic phase is washed with 4x80 ml of water and then dried over magnesium sulphate. 39 g of crude diphenylmethane-thiol are thus obtained. Yield 97.5%.
Benzhydryl-thioacetic acid
10 g (0.05 mol) of diphenylmethane-thiol and 2g (0.05 mol) of NaOH dissolved in 60 ml of demineralised water are introduced successively into a 250 ml flask equipped with a magnetic stirrer and a reflux condenser. The reactants are left in contact for 10 minutes whilst stirring, and a solution consisting of 7g (0.075 mol) of chloroacetic acid, 3g (0.075 mol) of NaOH pellets and 60 ml of demineralized water is then added all at once. The aqueous solution is gently warmed to about 50°C for 15 minutes, washed with 50 ml of ether, decanted and acidified with concentrated hydrochloric acid. after filtration, 10.2g of benzhydryl-thioacetic acid are thus obtained. Melting point 129-130°C. Yield 79%.
Ethyl benzhydryl-thioacetate
The following reaction mixture is heated under reflux for 7 hours: 10.2 g (0.0395 mol) of benzhydryl-thioacetic acid, 100 ml of anhydrous ethanol and 2 ml of sulphuric acid. When heating has been completed, the ethanol isevaporated in vacuo; the oily residue is taken up in 100 ml of ethyl ether and the organic solution is then washed with water, with an aqueous sodium carbonate solution and then with water until the wash waters have a neutral pH. After drying over sodium sulphate, the solvent is evaporated. 10.5g of ethyl benzhydryl- thioacetate are thus obtained. Yield 93%.
Benzhydryl-thioacetohydroxamic acid
The following three solutions are prepared:
- Ethyl Benzhydryl-thioacetate 10.8 g (0.0378 mol) in 40 ml methanol
- Hydroxylamine hydrochloride 5.25 g (0.0756 mol) in 40 ml methanol
- Potassium Hydroxide pellets 7.3 g (0.0134 mol) in 40 ml methanol
The solutions are heated, if necessary, until they become limpid, and when the temperatures have again fallen to below 40°C, the solution of potassium hydroxide in methanol is poured into the solution of hydroxylamine hydrochloride in alcohol. Finally, at a temperature of about 5° to 10°C, the solution of ethyl benzhydryl- thioacetate is added in its turn. After leaving the reactants in contact for 10 minutes, the sodium chloride is filtered off the limpid solution obtained is kept for about 15 hours at ordinary temperature. The methanol is then evaporated under reduced pressure, the residual oil is taken up in 100 ml of water and the aqueous solution is acidified with 3 N hydrochloric acid. The hydroxamic acid which has crystallized is filtered off, washed with water and then dried. 9.1 g of product are obtained. Yield = 87.5%. Melting point 118-120°C.
Adrafinil (CRL 40,028)
10.4g (0.038 mol) of benzhydryl-thioacetohydroxamic acid are oxidized at 40°C, over the course of 2 hours, by means of 3.8 ml (0.038 mol) of hydrogen peroxide of 110 volumes strength (33%), in 100 ml of acetic acid.
When the oxidation has ended, the acetic acid is evaporated under reduced pressure and the residual oil is taken up in 60 ml of ethyl acetate. The product which has crystallized is filtered off and then purified by recrystallisation from a 3:2 (by volume) mixture of ethyl acetate and isopropyl alcohol.
8g (73%) of Adrafinil, mp 159-160°C, are thus obtained. H2O Solubility <1 g/L.
CLIP
Figure 2: GC/MS extracted ion chromatogram (a) and mass spectrum (b) of derivatized adrafinil in the electron ionization mode (monitoring the m/z 167, 165 and 152 ions; all the four peaks are derivatised adrafinil products).
Figure 4: LC/ESI-MS full scan chromatogram of adrafinil and its metabolites (a) (modafinil acid RT 3.8 min, adrafinil RT 4.0 min, modafinil RT 4.1 min), and LC/ESI-MS full scan mass spectra of modafinil acid (b), adrafinil (c), and (d) modafinil. (b, c and d showing the similar ions at m/z 167, 165, 152 together with the appropriate sodium and potassium adducts).
NMR
Patent
FIG. 1 shows the structure of adrafinil and its metabolites.
FIG. 2 shows the chemical synthesis of adrafinil.
//////////////
References
- Jump up^ Robertson P, Hellriegel ET (2003). "Clinical pharmacokinetic profile of modafinil". Clin Pharmacokinet. 42 (2): 123–37. doi:10.2165/00003088-200342020-00002.PMID 12537513.
- ^ Jump up to:a b Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 20–. ISBN 978-3-88763-075-1.
- ^ Jump up to:a b c d e f g h i Milgram, Norton (1999). "Adrafinil: A Novel Vigilance Promoting Agent".CNS Drug Reviews. 5 (3): 193–212. doi:10.1111/j.1527-3458.1999.tb00100.x. Retrieved2 October 2014.
- ^ Jump up to:a b c d AFSSAPS (2011). "Point d'information sur les dossiers discutés en commission d'AMM Séance du jeudi 1er décembre 2011 - Communiqué".
- Jump up^ Simon P, Chermat R, Puech AJ (1983). "Pharmacological evidence of the stimulation of central alpha-adrenergic receptors". Prog. Neuropsychopharmacol. Biol. Psychiatry. 7 (2-3): 183–6. doi:10.1016/0278-5846(83)90105-7. PMID 6310690.
- Jump up^ Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, Prisinzano TE, Baumann MH (May 2009). "Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil". The Journal of Pharmacology and Experimental Therapeutics. 329 (2): 738–46. doi:10.1124/jpet.108.146142.PMC 2672878
 . PMID 19197004.
. PMID 19197004.
- Jump up^ Reith ME, Blough BE, Hong WC, Jones KT, Schmitt KC, Baumann MH, Partilla JS, Rothman RB, Katz JL (Feb 2015). "Behavioral, biological, and chemical perspectives on atypical agents targeting the dopamine transporter". Drug and Alcohol Dependence. 147: 1–19. doi:10.1016/j.drugalcdep.2014.12.005. PMC 4297708
 . PMID 25548026.
. PMID 25548026.
- Jump up^ Quisenberry AJ, Baker LE (Dec 2015). "Dopaminergic mediation of the discriminative stimulus functions of modafinil in rats". Psychopharmacology. 232 (24): 4411–9.doi:10.1007/s00213-015-4065-0. PMID 26374456.
- Jump up^ Ballas, Christos A; Deborah Kim; Claudia F Baldassano; Nicholas Hoeh (July 2002). "Modafinil: past, present and future". Expert Review of Neurotherapeutics. 2 (4): 449–57.doi:10.1586/14737175.2.4.449. PMID 19810941.
- Jump up^ Alan F. Schatzberg; Charles B. Nemeroff (2009). The American Psychiatric Publishing Textbook of Psychopharmacology. American Psychiatric Pub. pp. 850–. ISBN 978-1-58562-309-9.
- Jump up^ Ballas, Christos A; Kim, Deborah; Baldassano, Claudia F; Hoeh, Nicholas (2002). "Modafinil: past, present and future". Expert Review of Neurotherapeutics. 2 (4): 449–457.doi:10.1586/14737175.2.4.449. ISSN 1473-7175. PMID 19810941.
- ^ Jump up to:a b Jeffrey K Aronson (31 December 2012). Side Effects of Drugs Annual: A worldwide yearly survey of new data in adverse drug reactions. Newnes. pp. 6–. ISBN 978-0-444-59503-4.
- Jump up^ Thobois S, Xie J, Mollion H, Benatru I, Broussolle E (2004). "Adrafinil-induced orofacial dyskinesia". Mov. Disord. 19 (8): 965–6. doi:10.1002/mds.20154. PMID 15300665.
- ^ Jump up to:a b c d e f Antonio Guglietta (28 November 2014). Drug Treatment of Sleep Disorders. Springer. pp. 212–. ISBN 978-3-319-11514-6.
- ^ Jump up to:a b Jie Jack Li; Douglas S. Johnson (27 March 2013). Modern Drug Synthesis. John Wiley & Sons. pp. 2–. ISBN 978-1-118-70124-9.
- Jump up^ url=http://www.bloomberg.com/research/stocks/private/snapshot.asp?privcapId=1366624
- Jump up^ World Anti-Doping Agency - 2007 Prohibited List
- Jump up^ MCC Minutes Out of Session Meeting. Medsafe.govt.nz (2013-05-23). Retrieved on 2013-12-18.
External links
////////////ADRAFINIL
- O=S(C(c1ccccc1)c2ccccc2)CC(=O)NO