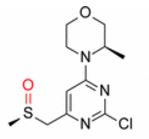
(3R)-4-[2-chloro-6-[[(R)-methylsulfinyl]methyl]pyrimidin-4-yl]-3-methyl-morpholine
Synthesis of (3R)-4-[2-chloro-6-[[(R)-methylsulfinyl]methyl]pyrimidin-4-yl]-3-methyl-morpholine (10)
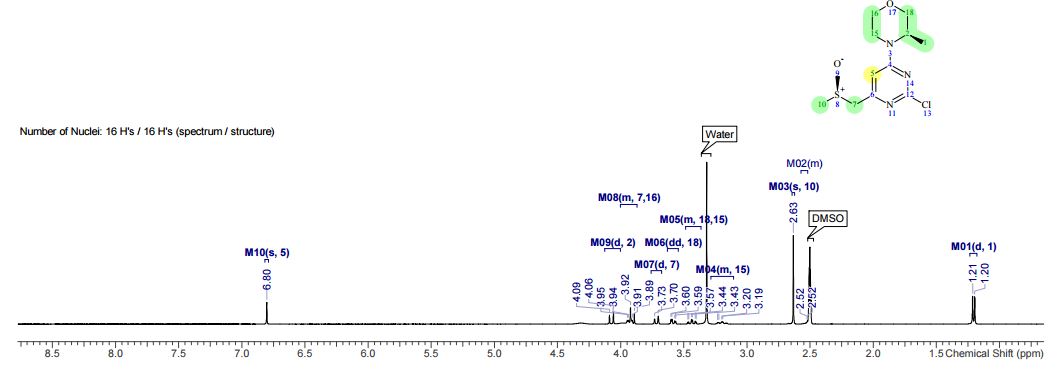
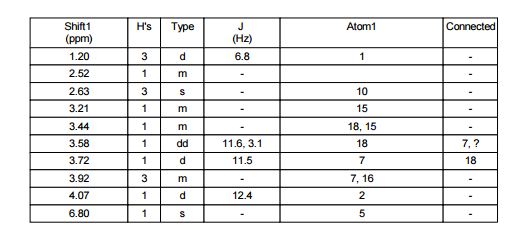
off-white solid (53.9 kg, 68.3% yield). 1H NMR (400 MHz, DMSO-d6, δ): 1.20 (d, J = 6.8 Hz, 3 H), 2.52 (m, 1 H), 2.63 (s, 3 H), 3.21 (m, 1 H), 3.44 (m, 1 H), 3.58 (dd, J = 11.6, 3.1 Hz, 1 H), 3.72 (d, J = 11.5 Hz, 1 H), 3.92 (m, 3 H), 4.07 (d, J = 12.4 Hz, 1 H), 6.80 (s, 1 H); Assay (HPLC) 99%; Assay (QNMR) 100%; Chiral purity (HPLC) (R,R)-diastereoisomer 99.6%, (R,S)-diastereoisomer 0.4%.

A Baeyer–Villiger monooxygenase enzyme has been used to manufacture a chiral sulfoxide drug intermediate on a kilogram scale. This paper describes the evolution of the biocatalytic manufacturing process from the initial enzyme screen, development of a kilo lab process, to further optimization for plant scale manufacture. Efficient gas–liquid mass transfer of oxygen is key to obtaining a high yield.
Development and Scale-up of a Biocatalytic Process To Form a Chiral Sulfoxide
The Departments of Pharmaceutical Sciences and Pharmaceutical Technology and Development, AstraZeneca, Silk Road Business Park, Macclesfield, Cheshire SK10 2NA, United Kingdom
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.6b00391
Publication Date (Web): January 4, 2017
Copyright © 2017 American Chemical Society
*Tel: +44 (0)1625-519149. E-mail: william.goundry@astrazeneca.com.

Examples of biologically active molecules containing a sulfoxide or sulfoximine: esomeprazole (3), aprikalim (4), oxisurane (5), OPC-29030 (6), ZD3638 (7), buthionine sulfoximine (8), and AZD6738 (9).
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This article is a compilation for educational purposes only.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent

No comments:
Post a Comment