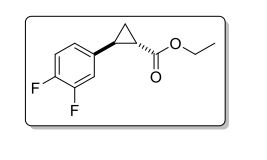
(±)-trans-ethyl 2-(3,4-difluorophenyl)Cyclopropanecarboxylate
C12H12F2O2
GC-MS (EI) m/z: [M]+ calc. for C12H12F2O2 + : 226.08; found: 226.08.
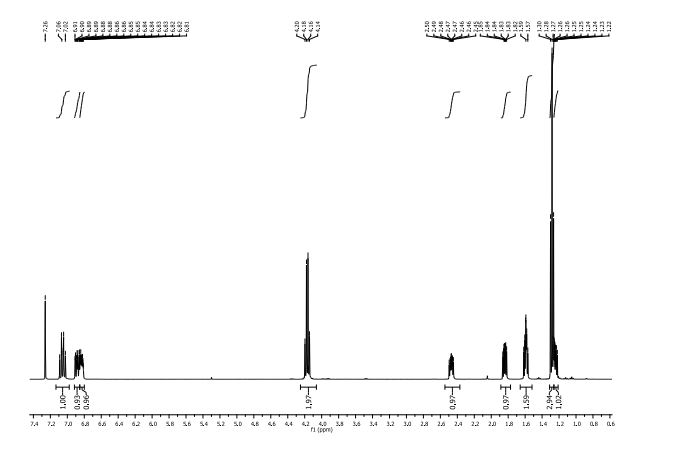
δH (400 MHz, CDCl3): 1.25 (1H, ddd, 3 J 8.4 Hz, 3 J 6.4 Hz, 2 J 4.5 Hz , 3-H); 1.28 (3H, t 3 J 6.4 Hz CH3Ethyl) 1.57-1.62 (2H, m, 3 J 9.2 Hz, 3 J 5.2 Hz, 2 J 4.5 Hz, 3-H + H2O), 1.84 (1H, ddd, 3 J 8.5 Hz, 3 J 5.3 Hz, 3 J 4.3 Hz , 2-H), 2.47 (1H, ddd, 3 J 9.5 Hz, 3 J 6.4 Hz, 3 J 4.2 Hz , 1-H), 4.17 (2H, q, 3 J 6.3 Hz, CH2Ethyl) 6.81-6.87 (1H, m, 3 J 8.5 Hz, 4 J 7.6 Hz, 4 J 2.4 Hz, 6-H’ ), 6.88 (1H, ddd, 3 J 11.5 Hz, 4 J 7.6 Hz, 4 J 2.2 Hz, 2-H’) 7.06 (1H, dt, 3 J 10.3 Hz, 3 J 8.2 Hz. 5-H’).
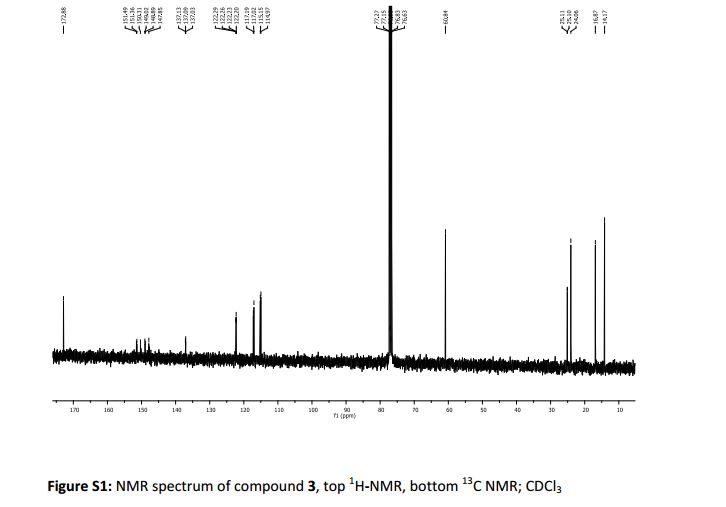
δc (400 MHz, CDCl3): 14.27 (CH3Ethyl), 16.84 (3-C) 24.04 (1-C), 25.14 (d, 4 J 1.4, 2-C), 60.71 (CH2Ethyl), 114.74 (d, 2 J 19 Hz, 2-C’), 117.09 (d, 2 J 18 Hz, 5-C’), 122.25 (dd, 3 J 6.1 Hz, 4 J 3.4 Hz, 6- C’), 137.06 (dd, 3 J 6.1 Hz, 4 J 3.4 Hz, 1- C’), 149.2 (dd, 1 J 248 Hz, 2 J 13 Hz, 4-C’) 151.32 (dd, 1 J 249 Hz, 2 J 12.5 Hz, 3-C’) 172.87 (Ccarbonyl).
[ ] 20 a D = -381.9 (c 1.0 in EtOH) for (1R,2R)-3, ee = 95%

In this study a batch reactor process is compared to a flow chemistry approach for lipase-catalyzed resolution of the cyclopropanecarboxylate ester (±)-3. (1R,2R)-3 is a precursor of the amine (1R,2S)-2 which is a key building block of the API ticagrelor. For both flow and batch operation, the biocatalyst could be recycled several times, whereas in the case of the flow process the reaction time was significantly reduced.
Comparison of a Batch and Flow Approach for the Lipase-Catalyzed Resolution of a Cyclopropanecarboxylate Ester, A Key Building Block for the Synthesis of Ticagrelor
† School of Chemistry, University of Manchester, Manchester Institute of Biotechnology, 131 Princess Street, Manchester M1 7DN, United Kingdom
‡ Chemessentia, SRL - Via G. Bovio, 6-28100 Novara, Italy
§ Institute of Process Research and Development, School of Chemistry, University of Leeds, Woodhouse Lane, Leeds, LS2 9JT, United Kingdom
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.6b00346
Publication Date (Web): December 22, 2016
Copyright © 2016 American Chemical Society
*E-mail: Nicholas.Turner@manchester.ac.uk.
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This article is a compilation for educational purposes only.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent

No comments:
Post a Comment