Cellulose acetate as a convenient intermediate for the preparation of 5-acetoxymethylfurfural from biomass
Abstract
5-Acetoxymethylfurfural (AMF) is an important biomass derived platform chemical related to 5-hydroxymethylfurfural. Such furanic compounds can be produced via the hydrolysis of cellulose followed by dehydration of the resulting glucose units. However, the integration of these reactions in a single process remains technically challenging, and the direct use of monosaccharides is often preferred. In this work we report a new method for the synthesis of AMF based on the acetolysis of cellulose acetate in the presence of sulfuric acid. The strategy was optimized for both batch and continuous processing. Furthermore, cellulose acetate prepared by direct wood acetylation could be successfully applied as a precursor, proving the method as a robust solution for integrated biomass processing.
Cellulose acetate as a convenient intermediate for the preparation of 5-acetoxymethylfurfural from biomass
Green Chem., 2017, Advance Article
DOI: 10.1039/C7GC00975E, Communication
 Open Access
Open Access This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.
Llorenc Gavila, Davide Esposito
A new method for the synthesis of AMF based on the acetolysis of cellulose acetate is reported. Cellulose acetate prepared by wood acetylation can be applied as a precursor, offering possibilities for integrated biomass processing
A new method for the synthesis of AMF based on the acetolysis of cellulose acetate is reported. Cellulose acetate prepared by wood acetylation can be applied as a precursor, offering possibilities for integrated biomass processing
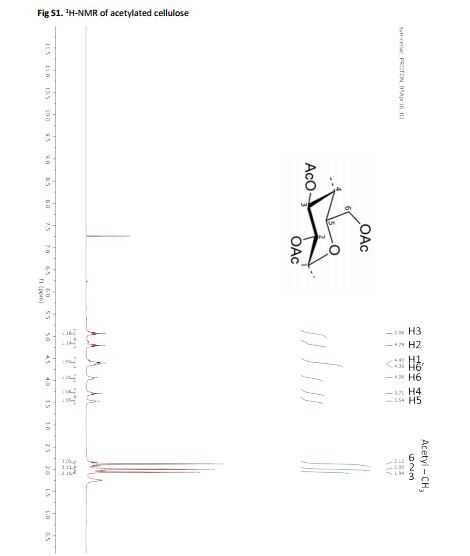
Cellulose acetate synthesis from cellulose In a round bottom flask, cellulose (2 g, 12 mmol) was suspended in acetic acid (35 mL) and stirred for 1 hour at 55 °C. Thus, a mixture of acetic anhydride (10 mL, 105 mmol) and sulfuric acid (0.4 mL, 7.5 mmol) was slowly added, while the mixture was kept at 55 °C for 2 hours3 . The mixture was thus poured into cold water, the precipitate was filtered, washed and dried at 40 °C in a vacuum oven.
Cellulose acetate synthesis from pulp or wood In a round bottom flask, 2 g of wood or pulp were suspended in acetic acid (35 mL) and stirred for 1 hour at 55 °C. Thus, a mixture of acetic anhydride (10 mL, 105 mmol) and sulfuric acid (0.4 mL, 7.5 mmol) was slowly added, while the mixture was kept at 55 °C for 2 hours3 . The mixture was poured into cold water, the precipitate was filtered, washed and dried at 40 °C in a vacuum oven. In order to purify the cellulose acetate, the precipitate was stirred in dichloromethane (30 mL) at 30 °C for 1 hour; afterwards 2/3 of the solvent was evaporated with a rotary evaporator and poured into 20 mL of ethanol, the precipitate was washed with ethanol and dried at 40 °C in a vacuum oven.
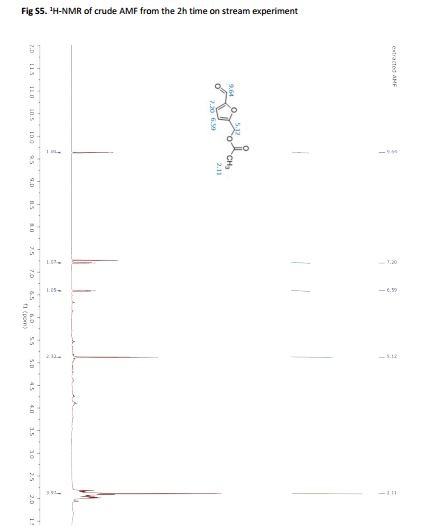
General procedure for the acetolysis of cellulose acetate Solutions of cellulose acetate in acetic acid were prepared under the approximation that all cellulose acetate is composed of triacetylated anhydroglucose units (molar mass: 288 g/mol). The following molarities (mM) and the number of equivalents are therefore calculated with respect to triacetylated anhydroglucose units. Briefly, the corresponding amount of cellulose acetate to reach a final concentration of 5 g/L (17.4 mM) of cellulose acetate were dissolved in acetic acid and 35 mM of acetic anhydride (2 eq) and 35 mM of acid (2 eq) was added each run, unless otherwise stated. (The same procedure was adapted for non-acetylated cellulose [molar mass: 162 g/mol] as control experiment).

No comments:
Post a Comment