
N-Methyl-3-Bromo-5-Methyl Pyrazole
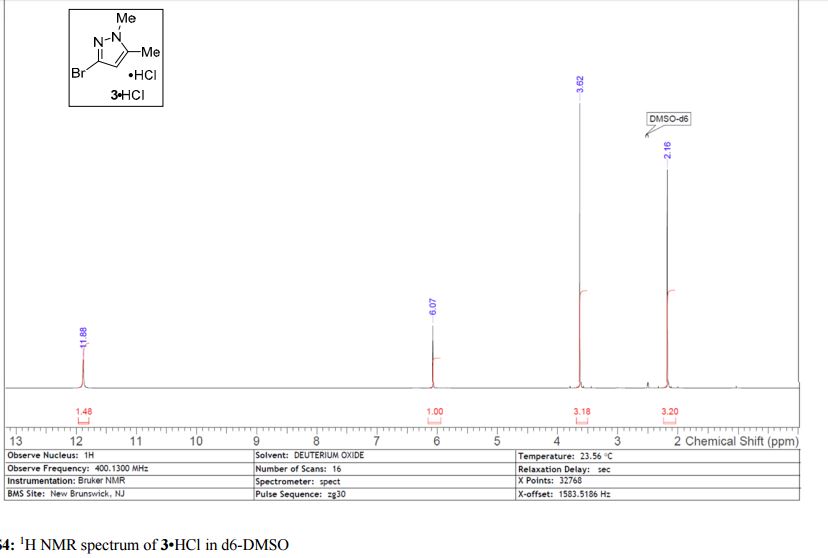
3·HCl as a white solid in 27% yield; sublimes at 40 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.88 (s, 1 H), 6.07 (s, 1 H), 3.62 (s, 3 H), 2.16 (s, 3 H); 13C NMR (125 MHz, DMSO-d6) δ 141.7, 123.0, 107.5, 36.5, 10.9; HRMS-ESI (m/z) calcd for C5H8N2Br [M + H]+ 174.9864, found 174.9864.
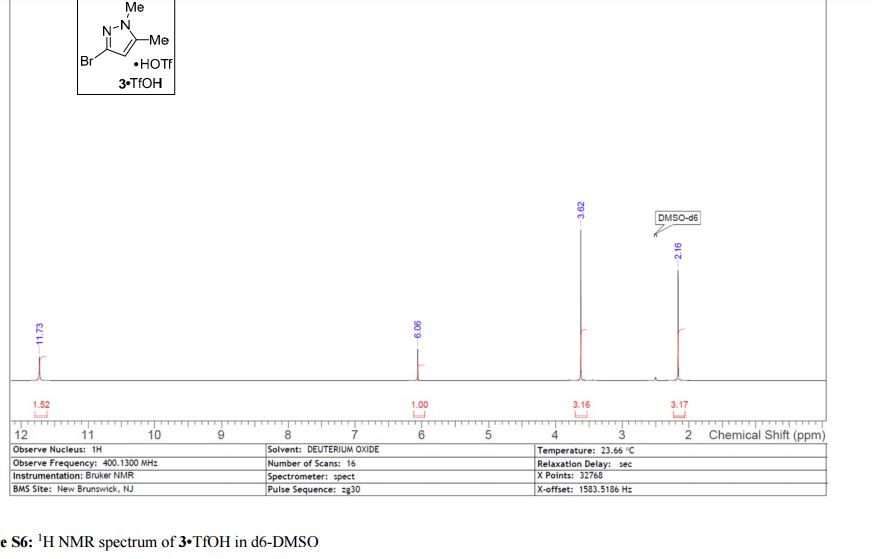
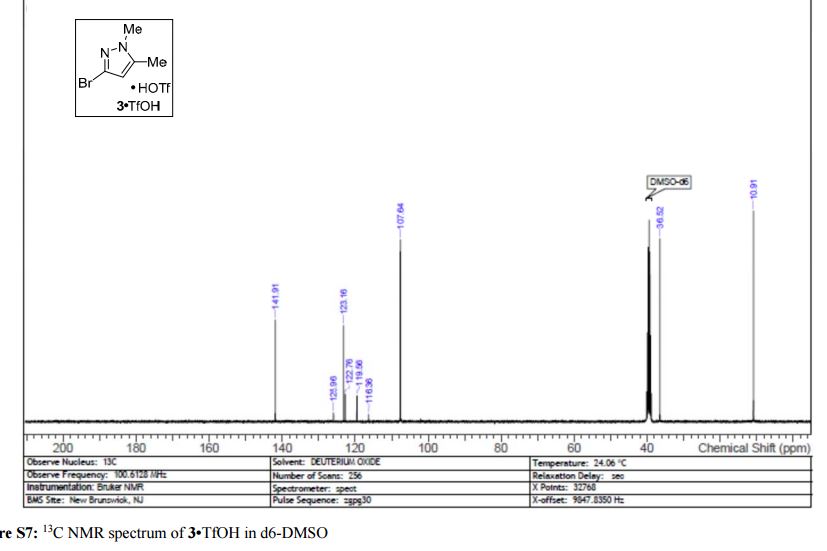
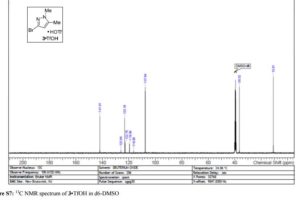
3·TfOH as an off-white solid; mp = 145 °C; 1H NMR (400 MHz, DMSO-d6) δ 11.73 (s, 1 H), 6.06 (s, 1 H), 3.62 (s, 3 H), 2.16 (s, 3 H); 13C NMR (100 MHz, DMSO-d6) δ 141.9, 123.2, 121.2 (q, J = 320 Hz), 107.6, 36.5, 10.9; HRMS-ESI (m/z) calcd for C5H8N2Br [M + H]+ 174.9864, found 174.9865.
Development of Scalable Processes for the Preparation of N-Methyl-3-Bromo-5-Methyl Pyrazole
Chemical & Synthetic Development, Bristol-Myers Squibb Company, P.O. Box 191 New Brunswick, New Jersey 08903-0191, United States
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.7b00091
*E-mail: richard.fox@bms.com.
The development and optimization of two scalable routes to N-methyl-3-bromo-5-methyl pyrazole is described. The initial Sandmeyer route entailed a three-step sequence from crotonitrile and methyl hydrazine, proceeding through the 3-amino pyrazole intermediate. Due to the GTI liability of the 3-amino pyrazole intermediate, a tedious steam-distillation, and <30% overall yield, we developed a second-generation Sandmeyer-free approach from methyl crotonate and methyl hydrazine which leveraged a condensation, bromination, and oxidation sequence. Process development led to the improved preparation of N-methyl-3-bromo-5-methyl pyrazole with increased efficiency and overall yield. The isolation, handling, and storage of the final product was greatly improved through the generation of the triflic acid salt, and salt form studies are also discussed.
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.7b00091
/////////////




No comments:
Post a Comment