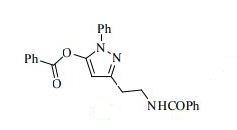
3-(2-(Benzoylamino)ethyl)-1-phenyl-1H-pyrazol-5-yl benzoate (14). Benzoyl chloride (0.230 mL, 2 mmol) was added to a stirred suspension of amine 6c (276 mg, 1 mmol) in a mixture of anhydrous dichloromethane (10 mL) and 4-methylmorpholine (0.66 mL, 6 mmol) and the mixture was stirred at r.t. for 12 h. Volatile component were evaporated in vacuo and the residue was chromatographed over silica gel (50% EtOAc/hexanes). Fractions containing the products were combined and evaporated in vacuo to give 14. Yellow solid, yield 79%, 326 mg,
mp 109– 112 °C,
IR (νmax, cm –1): 3289, 1753 (C=O), 1632 (C=O), 1445, 1315, 1247, 1076, 760, 697.
1 H NMR (300 MHz, CDCl3), δH 3.01 (2H, t, 3 JHH = 6.3 Hz, CH2CH2NH), 3.86 (2H, q, 3 JHH = 6.0 Hz, CH2CH2NH), 6.36 (1H, s, 4-H of pyrazole), 7.16 (3H, br s, NH), 7.31–7.53 (8H, m, 8H of Ph), 7.59–7.68 (3H, m, 3H of Ph), 7.78–7.83 (2H, m, 2H of Ph), 8.05–8.11 (2H, m, 2H of Ph).
13C NMR (126 MHz, DMSO-d6), δC 28.7, 38.9, 95.8, 122.7, 127.17, 127.23, 127.3, 128.3, 129.25, 129.30, 130.1, 131.1, 134.6, 134.8, 137.6, 144.0, 150.3, 161.8, 166.2. MS, m/z = 412 (MH+ ),
HRMS (ESI), m/z = 412.1649 (MH+ ), C25H22N3O3 requires 412.1656.
Anal. Calcd for C25H21N3O3 (411.45): C, 63.66; H, 6.16; N, 17.13%, Found: C, 63.47; H, 6.30; N, 16.91%.
Synthesis of 3-(2-aminoethyl)-5-hydroxy-1H-pyrazole derivatives
Volume 2012, Issue 3, Commemorative Issue in Honor of Prof. Rainer Beckert on the occasion of his 60th anniversary, pp. 49-65
No comments:
Post a Comment