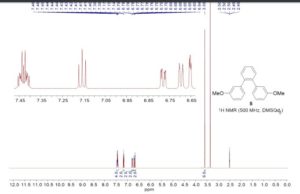
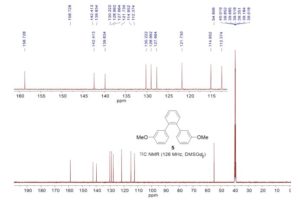
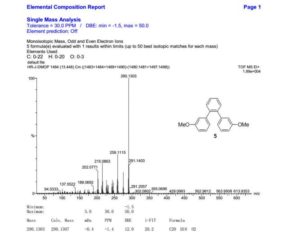
1,2-Bis(3-methoxyphenyl)benzene (5)
1H NMR (500 MHz, DMSO-d6): δ 3.59 (s, 6H), 6.65 (dd, J = 2.5, 1.5 Hz, 2H), 6.70 (ddd, J = 7.5, 1.5, 1.0 Hz, 2H), 6.78 (ddd, J= 8.0, 2.5, 1.0 Hz, 2H), 7.16 (t, J = 8.0 Hz, 2H), 7.40–7.46 (m, 4H).
13C NMR (126 MHz, DMSO-d6): δ 54.8, 112.4, 115.0, 121.7, 127.7, 129.0, 130.2, 139.8, 142.4, 158.7.
HRMS (TOF MS EI+) for C20H18O2 [M]+: calcd 290.1307, found 290.1303.
Efficient and Practical Synthesis of Electron Transport Material and Its Key Intermediate
† State Key Laboratory Breeding Base of Green Chemistry-Synthesis Technology, College of Chemical Engineering, Zhejiang University of Technology, 18 Chaowang Road, Hangzhou, Zhejiang 310014, P. R. China
‡ Department of Materials Science and Engineering, Arizona State University, Tempe, Arizona 85284, United States
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.7b00280
*E-mail: guijieli@zjut.edu.cn., *E-mail: sheyb@zjut.edu.cn.
Abstract
An efficient and practical synthesis of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)triphenylene 4 from two cheap commodity chemicals in five steps with a total yield of 48.6% was developed. This process had been successfully applied in the synthesis of electron transport material (ETM) BPyTP-2 in the gram scale with a total yield of 47.2%. This practical development of the key intermediate 4 opens a door in its further application in the synthesis of other triphenylene-based ETMs and host materials in the materials field.
/////////////http://pubs.acs.org/doi/10.1021/acs.oprd.7b00280
No comments:
Post a Comment