Unconventional Method for Synthesis of 3-Carboxyethyl-4-formyl(hydroxy)-5-aryl-N-arylpyrazoles
† Departamento de Química, Universidade Estadual de Maringá (UEM), 87030-900 Maringá, PR, Brazil
‡ Departamento de Química, Universidade Federal de Santa Maria (UFSM), 97110-970 Santa Maria, RS, Brazil
§ Instituto de Biotecnologia, Universidade de Caxias do Sul (UCS), 295070-560 Caxias do Sul, RS, Brazil
J. Org. Chem., 2017, 82 (23), pp 12590–12602
DOI: 10.1021/acs.joc.7b02361
Publication Date (Web): November 2, 2017
*E-mail: farosa@uem.br
Cite this:J. Org. Chem. 82, 23, 12590-12602
Abstract
An alternative highly regioselective synthetic method for the preparation of 3,5-disubstituted 4-formyl-N-arylpyrazoles in a one-pot procedure is reported. The methodology developed was based on the regiochemical control of the cyclocondensation reaction of β-enamino diketones with arylhydrazines.
Structural modifications in the β-enamino diketone system allied to the Lewis acid carbonyl activator BF3 were strategically employed for this control. Also a one-pot method for the preparation of 3,5-disubstituted 4-hydroxymethyl-N-arylpyrazole derivatives from the β-enamino diketone and arylhydrazine substrates is described.

4-Formyl-N-arylpyrazole substrates occupy a prominent position in the field of organic synthesis since they are key intermediates in obtaining a wide range of biologically active compounds. Because of the synthetic versatility of the 4-formyl-N-arylpyrazole skeleton, their synthesis has been extensively explored. In an extension of their previously published research,
Rosa and co-workers at Universidade Estadual de Maringá described a one-pot synthetic method that regioselectively produced 3,5-disubstituted-4-formyl-N-arylpyrazoles . The β-enamino diketone starting materials were readily synthesized via published procedures. High regioselectivity was secured via the use of BF3·OEt2 as the carbonyl activator and a bulky amine as the enamine component. Acetonitrile proved to be the most suitable solvent for the reaction.
After an aqueous workup, the desired pyrazoles were obtained in excellent yields. A variety of functional groups were tolerated on the two aryl substituents. This operationally simple procedure afforded the 4-formyl-N-arylpyrazoles in high yields, regioselectively. Furthermore, the formyl group could be reduced in situ with sodium borohydride to generate the corresponding 4-hydroxymethyl-N-arylpyrazoles.
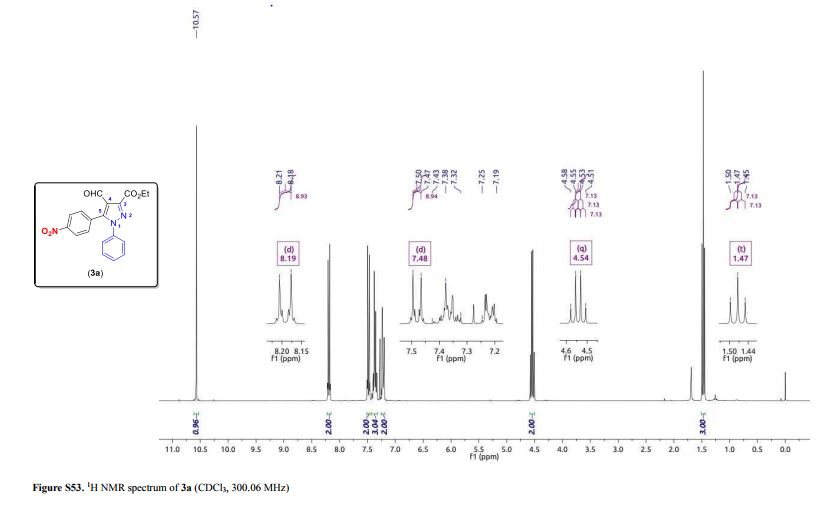
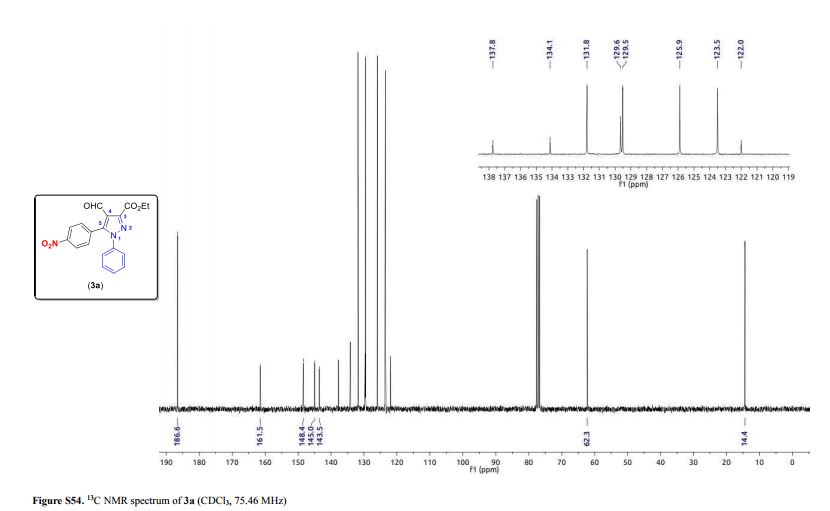
3-(Ethoxycarbonyl)-4-formyl-5-(4-nitrophenyl)-1-phenyl-1H-pyrazole (3a)
Light yellow solid; yield: 0.150 g (82%); mp 147.0–149.2 °C;
1H NMR (300.06 MHz, CDCl3) δ (ppm) 1.47 (t, 3H, J = 7.1 Hz, O–CH2–CH3), 4.54 (q, 2H, J = 7.1 Hz, O–CH2-CH3), 7.19–7.25 (m, 2H, Ph), 7.32–7.43 (m, 3H, Ph), 7.48 (d, 2H, J = 8.9 Hz, 4-NO2C6H4), 8.19 (d, 2H, J = 8.9 Hz, 4-NO2C6H4), 10.57 (s, 1H, CHO);
13C NMR (75.46 MHz, CDCl3) δ (ppm) 14.4 (O–CH2–CH3), 62.3 (O-CH2–CH3), 122.0 (C4), 123.5 (4-NO2C6H4), 125.9 (Ph), 129.5 (Ph), 129.6 (Ph), 131.8 (4-NO2C6H4), 134.1 (4-NO2C6H4), 137.8 (Ph), 143.5 (C5), 145.0 (C3), 148.4 (4-NO2C6H4), 161.5 (COOEt), 186.6 (CHO);
HRMS (ESI+): calcd for C19H16N3O5+, [M+H]+: 366.1084, found 366.1101.


No comments:
Post a Comment