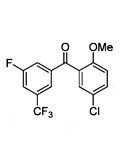
Cas 329941-92-6
C15 H9 Cl F4 O2
Methanone, (5-chloro-2-methoxyphenyl)[3-fluoro-5-(trifluoromethyl)phenyl]-
(5-Chloro-2-methoxyphenyl)[3-fluoro-5-(trifluoromethyl)phenyl]methanone (7)
7 as white crystals
mp 93–95 °C;
1H NMR (500 MHz, CDCl3) 7.82 (s, 1H), 7.66 (d, J = 8.5 Hz, 1H), 7.53 (d, J =7.5 Hz, 1H), 7.49 (d, J = 9.0 Hz, 1H), 7.42 (s, 1H), 6.97 (d, J = 9.0 Hz, 1H), 3.71 (s, 3H);
13C NMR (500 MHz, CDCl3) 192.1, 162.4 (d, J = 249.3 Hz), 156.0, 140.4 (d, J = 6.4 Hz), 132.8, 132.7 (dq, J = 7.5, 33.6 Hz), 129.7, 128.4, 126.3, 122.9 (q, J = 272.3), 122.1 (m), 119.5 (d, J = 22.4), 116.9 (m), 113.3, 55.3;
HRMS calculated for C15H9ClF4O2 [M + H]+: 333.0299, Found: 333.0306.



A convergent synthesis of NNRTI 1 is described. The key step involves a direct coupling of acid chloride 4 with Grignard reagent 11 in the presence of bis[2-(N,N-dimethylamino)ethyl] ether that moderates the reactivity of the Grignard reagent to give benzophenone 7. An efficient 2-step process for the preparation of 2-fluoro-3-methyl-4-aminobenzoic acid (3) is also described.
Practical Synthesis of A Benzophenone-Based NNRT Inhibitor of HIV-1
† Chemical Development, Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, Connecticut 06877, United States
‡ Boehringer Ingelheim (Canada) Ltd., Research and Development, 2100 Cunard Street, Laval, Québec H7S 2G5, Canada
Org. Process Res. Dev., 2012, 16 (4), pp 561–566
DOI: 10.1021/op200301h
*E-mail: xiao-jun.wang@boehringer-ingelheim.com.
///////