tert-butyl(3aR,6aS)-5-oxohexahydrocyclopenta[c]pyrrole-2(1H)-carboxylate
tert-Butyl (3aR,6aS)-5-Oxohexahydrocyclopenta[c]pyrrole-2(1H)-carboxylate (1)
pure compound 1 (1.051 kg, 67%) as a white solid. Mp: 70–71 °C (uncorrected); [α]25D +0.40° (c 1.00 CHCl3); % purity: 98.5% (HPLC);
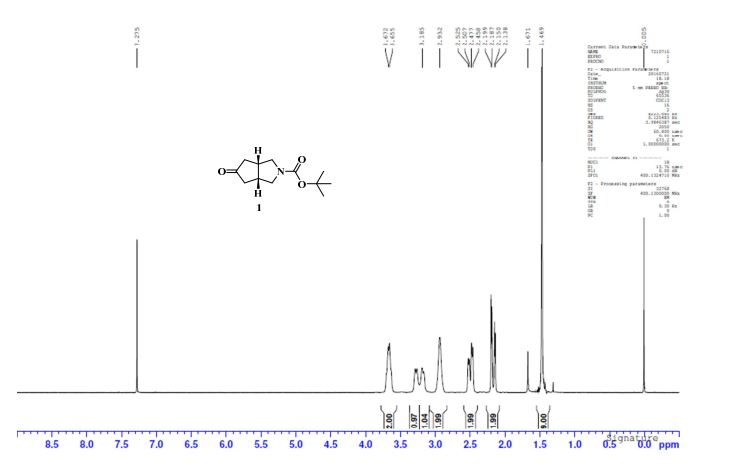
1H NMR (CDCl3, 400 MHz) δ: 1.46 (s, 9H), 2.15 (dd, J1 = 4.8 Hz, J2 = 19.6 Hz, 2H), 2.47 (dd, J1 = 7.4 Hz, J2 = 19.6 Hz, 2H), 2.93 (bs, 2H), 3.16–3.28 (m, 2H), 3.65–3.67 (m, 2H).;
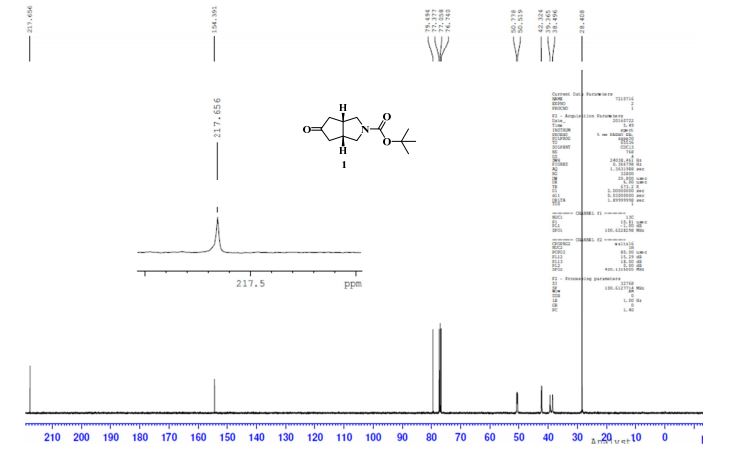
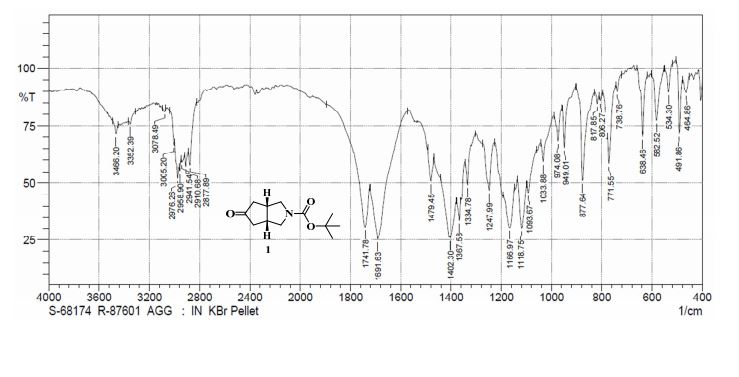
13C NMR (CDCl3, 100 MHz) δ: 38.49, 39.36, 42.32, 50.51, 50.77, 79.49, 154.39, 217.65; IR (KBr): ν = 638, 771, 877, 1118, 1166, 1247, 1367, 1402, 1691, 1741, 2877, 2910, 2958, 2976, 3005 cm–1;
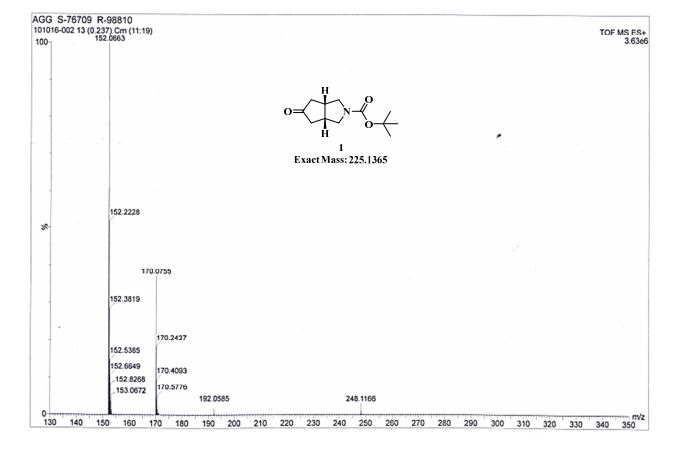
TOFMS: [C12H19NO3 + H+]: calculated 226.1438, found 152.0663 (M-OtBu)+ (100%), 170.0755 (M-tBu + H)+ (40%), 248.1166 (M + Na)+ (5%).
Anal. Calcd for C12H19NO3: C, 63.98; H, 8.50; N, 6.22. Found: C, 63.89; H, 8.27; N, 5.97.
HPLC conditions were as follows for compound ; Agilent 1100 series, column: YMC J’SPHERE C18 (150 mm X 4.6 mm) 4µm with mobile phases A (0.05% TFA in water) and B (acetonitrile). Detection was at 210 nm, flow was set at 1.0 mL/min, and the temperature was 30 °C (Run time: 45 min). Gradient: 0 min, A = 90%, B = 10%; 5.0 min, A = 90%, B = 10%; 25 min, A = 0%, B = 100%; 30 min, A = 0%, B = 100%, 35 min, A = 90%, B = 10%; 45 min, A = 90%, B = 10%.
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.6b00399
/////