BOCEPREVIR
Handelsname: Victrelis®,
Patentnummer: WO2002008244
CAS394730-60-0
N-[3-amino-1-(cyclobutylmethyl)-2,3-dioxopropyl]-3-{N-[(tert-butylamino)carbonyl]-3-methyl-L-valyl}-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide
Hepatitis C virus (HCV) chronically infects more than 200 million
people worldwide, and current treatment options have been very limited.
Boceprevir, a protease inhibitor, which is a drug molecule approved in
2011, is useful for the treatment of human hepatitis C virus infections.
It is an amorphous mixture of two diastereomers in the ratio 1.15:1,
which differ in their stereochemical configuration at the third carbon
atom from the ketoamide end of the molecule. Boceprevir is used in
combination with interferon α-2b and ribavirin in the treatment of
chronic HCV genotype 1 infection.
PAPER
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/op500065t
Efforts toward the synthesis and process optimization of boceprevir
1 are
described. Boceprevir synthesis was optimized by telescoping the first
three steps and last two steps of the five-step process. Optimization of
oxidation, which is one of the critical steps in the total synthesis,
is discussed. A control strategy for the three impurities is described. A
novel process for the synthesis of fragment A (
2) has been developed, which is the key starting material for the synthesis of boceprevir.
…………………


WO 2015004685
( 1 R,5S)-N-[3-Amino- 1
-(cyclobutylmethyl)-2,3-dioxopropyl]-3-[2(S)-[[[( 1 , 1 -dimethylethyl)
amino]carbonyl]amino]-3,3-dimethyl-l-oxobutyl]-6,6-dimethyl-3-azabicyclo
[3.1.0]hexan-2(S)-carboxamide (Boceprevir); having formula I. It is a
hepatitis C virus (“HCV”) protease inhibitor, developed by Merck &
Co and marketed under the brand name of VICTRELIS.

Formula I
U.S. patent number 6,992,220, U.S. patent application numbers 201
1034705, U.S. 20050249702 and U.S. 201001 13821 are disclosed process
for the preparation of Boceprevir.
U.S. patent number 7,326,795 claims Boceprevir bisulfate adduct as a
product. Advanced Organic Chemistry, 4th ed., Jerry March Ed., John
Wiley and Sons, 1972 disclosed purification methods from bisulfate
adduct to provide the compound in a pure form.
U.S. patent number 8,222,427 claims a process for the purification of
Boceprevir through a corresponding bisulfite adduct, wherein the
compound of Formula I is dissolved in organic solvent, which is treated
with an aqueous phase comprising bisulfite, thereby forming an aqueous
solution of the bisulfite adduct of the compound of Formula I, which is
subsequently regenerated from the aqueous phase without isolating the
bisulfite adduct.

Examples:
Example 1:
183.7 gm of l-Dimethylaminopropyl-3-ethylcarbodiimide hydrochloride
and 500 ml of dimethylsulfoxide were taken at 23-25 °C and to this 500
ml of ethyl acetate was added then cooled to 2-8 °C.
3-[2-(3-Tert-butylureido)-3,3-dimethyl-butyryl]-6,6-dimethyl-3-azabicyclo[3.1.0]
hexane-2 carboxylic
acid(2-carbamoyl-l-cyclobutyl-(methyl-2-hydroxy-ethyl)amide (Hydroxy
Boceprevir) 100 gm was added to the reaction mixture under stirring at
same temperature followed by 86.5 gm of dichloroacetic acid and
continued stirring for 1-2 hrs. After completion of the reaction, 2500
mL of water was added to the reaction mixture at 2-10 °C and the
reaction mixture temperature was raised to 15-20 °C. Ethyl acetate 600
ml was added to the reaction mass and the organic layer was separated.
The product was extracted from aqueous layer with ethyl acetate. The
organic layer was washed with 5% w/w hydrochloric acid followed by
water. To the organic layer, aqueous solution of sodium bisulfite (300
gm in 600 ml) was added and stirred for 2 hrs. The layers were separated
and organic layer was extracted with water. Thereafter, extracted
aqueous layer was washed with ethyl acetate. To the aqueous layer sodium
bisulfite (5.1 gm in 17 ml of water) was added and stirred for 30 min.
The obtained solution was degassed and the pH was adjusted to 1.0 to 2.5
with dilute hydrochloric acid (15 ml of 35% w/w hydrochloric acid and
15 ml of water) and cooled to 10-15 °C. The obtained solid was filtered
and washed with water to yield pure Boceprevir.
Exam le 2:
202 gm of l-Dimethylaminopropyl-3-ethylcarbodiimide hydrochloride and
500 ml of dimethylsulfoxide were taken at 23-25 °C and stirred, to this
reaction mixture 500 ml of ethyl acetate was added; stirred and cooled
to 2-8 °C. Hydroxy Boceprevir 100 gm was added under stirring at same
temperature followed by 92.7 gm of dichloroacetic acid and continued
stirring for 2-4 hrs. After completion of the reaction, 2500 mL of water
was added to the reaction mixture at 2-10 °C and temperature was raised
to 20-25 °C. Ethyl acetate 600 ml was added to the reaction mass and
the organic layer was separated. The product was extracted from aqueous
layer with ethyl acetate. The both organic layers were combined and
stirred with dilute hydrochloric acid solution (prepared by mixing 50 ml
of ~35% w/w of hydrochloric acid and 950 mL of water). The organic
layer containing the product was separated and washed with water. The
organic layer was cooled to 1-5 °C. To the organic layer, aqueous
solution of sodium bisulfite (300 gm in 600 ml) was added and stirred
for 2 hrs at 5- 9 °C. The organic layer was cooled without agitation and
added precooled water at 5-10 °C. The aqueous layer containing the
product was collected. The aqueous layer filtered through hyflo and
washed with precooled water. Further the aqueous layer was diluted with
precooled water, and adjusted the pH to 2 – 2.8 with dilute hydrochloric
acid. Vacuum was applied to the aqueous layer and the temperature was
slowly raised to less than 23 °C under reduced pressure. The separated
solid was filtered at 22-30 °C and washed with water. Further, the
filtered solid was washed with water having pH 1.8-2.4 (The pH of the
water was adjusted with HC1). The product was dried at 24-28 °C under
reduced pressure to yield pure Boceprevir.
Example 7:
100 gm of Crude Boceprevir was added to 300 mL of ethanol-isopropyl
alcohol (1 : 1) at 22-30 °C and contents were stirred for about 40
minutes. The resulting solution was added to water slowly at 5-10 °C and
stirred for 2-4 hrs at the same temperature. The product was filtered,
washed with water and dried at 25-30°C under reduced pressure.

…………………
SCHERING CORPORATION Patent: WO2008/76316 A2, 2008 ; Location in patent: Page/Page column 27 ;
or eq
https://www.google.co.in/patents/EP2121604A2?cl=en
Hepatitis C virus (HCV) is a (+)-sense single-stranded RNA virus that
has been implicated as the major causative agent in non-A, non-B
hepatitis; an HCV protease necessary for polypeptide processing and
viral replication has been identified. U.S. Patent No. 7,012,066
discloses a genus of HCV protease inhibitor compounds that includes the
compound of Formula I, (1 R,5S)-N-[3-amino-1-(cyclobutylmethyl)-2,3-
dioxopropyl]-3-[2(S)-[[[(1 , 1
-dimethylethyl)amino]-carbonyl]amino]-3,3-dimethyl-1 –
oxobutyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexan-2(S)-carboxamide.
Formula I
US2005/0059800, published March 17, 2005, discloses a process for
preparing the compound of Formula I and discloses a bisulfite adduct of
Formula I which can be used to provide the compound in a pure form in
accordance with the methods taught in Advanced Organic Chemistry, 4
th ed., Jerry March Ed., John Wiley and Sons, 1972.
US2005/0020689, filed January 27, 2005, discloses processes for
preparing an intermediate useful in preparing the compound of Formula I.
Methods for preparing diastereomers of the compound of Formula I are
disclosed in US2005/0249702, filed November 10, 2005. Published US
Patent Application No. 2007/0149459, filed November 13, 2006, discloses
oxidation processes for preparing the compound of Formula I.
Purification of the compound of Formula I is difficult for several
reasons. The compound Formula I is an alpha-keto amide that is unstable
and forms dimers, especially under basic conditions. Also, the compound
of Formula I is amorphous, thus it does not crystallize and
precipitation does not improve the purity of the solid —
Previously published procedures for preparing the compound of Formula I resulted in about 63 to about 98.5% purity.
Historically, aldehydes and ketones have been purified by preparing
their bisulfite adduct. Bisulfite purification of these types of
compounds was performed through isolation of a solid bisulfite adduct
intermediate from aqueous alcoholic solution by filtration. Regeneration
of an aldehyde or ketone from an isolated bisulfite adduct is
accomplished using a base or a strong acid. Examples appearing in the
literature of regeneration using bases includes: Na
2Cθ
3 in Org. Synthesis Coll. Vol. 4, 903 (1963); NaOH in WO 2006/074270 A2; and K
2CO
3 in Tetrahedron Lett., 45, 3219 (2004). Examples of regeneration using acids include: H
2SO
4 in J. Am. Chem. Soc, 70, 1748 (1948); and HCI in WO 99/57123.
For the preparation of a purified product, isolation of an
intermediate solid bisulfite adduct is not preferred since filtration of
the adduct is required. In addition, base regeneration of the adduct to
yield the substrate is not appropriate in those cases wherein the
regenerated product is unstable in basic conditions, for example, where
the regenerated product is the compound of Formula I. When acid
conditions are used to regenerate the substrate compound from a
bisulfite adduct, generally strongly acidic conditions and heating are
necessary (see references above).
Published international application no. WO 99/57123 reports using
non- alcoholic solvent in a process for forming a bisulfite adduct,
however the process required isolation of a solid bisulfite adduct and
regeneration the substrate from the adduct using NaOH.
A non-aqueous method for regeneration of a substrate from the
corresponding bisulfite adduct was reported in J. Org. Chem., 64, 5722
(1999) as a means to overcome side-reactions such as degradation and
hydrolysis during regeneration of aldehyde/ketone with a base or an
acid. In this method, trimethylsilyl chloride (TMSCI) or its equivalent
was employed in acetonitrile. During the process TMS
2O, NaCI
1 SO
2 and HCI were generated as co-products when TMSCI was used.
Removal of the co-products required the process steps of filtration
(for NaCI), aqueous work-up (for NaCI and excess TMSCI) and distillation
(for TMS
2O), which requires use of a high boiling solvent.
Regeneration of aldehydes from the corresponding bisulfite adducts with
ammonium acetate in solvent-free conditions was reported in J. of Chem.
Research, 237 (2004), however this process requires microwave
irradiation.
Published international application no. WO 2006/076415 describes
regeneration of an aldehyde from a corresponding bisulfite adduct
isolated from an alcoholic solvent system using a carbonate base with a
lower alkyl carbonyl compound, for example, acetone and glyoxylic acid.
SCHEME Il
solv
Bisulfite Adduct
Formula I in water Formula I
SCHEME III
Formula I
Published U.S. patent application no. 2007/0149459, published June
28, 2007, discloses several alternate procedures for oxidizing the
intermediate compound of the Formula II:
Formula II, to obtain the compound of Formula I.
HPLC Determination of Purity
The purity of the compound of Formula I is determined by HPLC according to the methods described below:
alternatively, the following equipment and conditions are used:

Example 1
(Purification Process of Scheme III, Regeneration Option “a”)
Preparation of Compound: To a reactor was charged (16.5 kg) of the compound of Formula II,
Formula Il24.3 Kg of EDCI
1 and 190 L of EtOAc. The batch temperature was adjusted between 15 and 25
0C. At the same temperature, Et
3N
(9.60 kg, 3 eq) followed by EtOAc rinse (8 L) was charged. To the
resultant mixture was charged DMSO (83 L) while maintaining the
temperature of the batch between 15
0C and 25
0C. CH
3SO
3H (10.89 kg) was charged while maintaining the reaction mixture between 15
0C and 30° C. After agitating at the reaction mixture for 1.5 hours while maintaining the reaction mixture between 20
0C and 30
0C, the reaction mixture was cooled to a temperature between -5
0C and 5
0C.
Purification of the Compound of Formula I
In a separate reactor was charged 165 L of water and 33 L of EtOAc, and the mixture was cooled below 5
0C. The reaction mixture containing the compound was transferred into the mixture of cold water/EtOAc at 0 to 10
0C.
The organic layer was separated and washed with water (99 L) three
times. Step 1 : To the resulting organic solution was added NaHSθ3
aqueous solution
(prepared from 49.5 kg of NaHSO
3 and 109 L of water). The whole was agitated for 3 h at 20-30
0C. The aqueous NaHSO
3 layer
was separated and saved. The organic layer was concentrated to about
116 L of volume and diluted with MTBE (220 L). The separated aqueous
NaHSO
3 layer was added to the organic layer. The resultant mixture was agitated for 3 h at 20-30
0C. The organic layer was separated and cooled to 0-10
0C.
Step 2: To the cooled organic layer of Step 1 was added cold water (165 L, 0-10
0C)
without agitation, and the whole was agitated for 5 min. The aqueous
layer was separated, and a solution of water (2 L) containing NaHSO
3 (0.71 kg) was added to the water layer. The water layer was distilled to the final volume of about 171 L under vacuum below 25
0C to remove volatiles.
Step 3: (Regeneration method a): The resultant water layer of Step 2
was added into a slurry of NaCI (49.5 kg) in acetone (83 L) at 20-30
0C.
The separated acetone layer followed by acetone rinse (8 L) was added
through a 0.2 micron filter to water (347 L) over 20 min at 15-25
0C.
After agitation for about 1 h, the precipitate was filtered and washed
with water (83 L). The wet cake was dried under vacuum at 30-40
0C to produce 13.0 kg (79%) of the purified compound as a white solid.

………………..
US2007/149459
http://www.google.co.in/patents/US20070149459
EXAMPLESPreparation of
(1R,2S,5S)-N-[3-amino-1-(cyclobutylmethyl)-2,3-dioxopropyl]-3-{N-[(tert-butylamino)carbonyl]-3-methyl-L-valyl}-6,6-dimethyl-3-azabicyclo-[3.1.0]hexane-2-carboxamide
(the Compound of Structure 2 in Scheme A, Below)
Example 1Preparation of Compound 2 Using Aqueous Acetic Acid in the Reaction Mixture
Into a 1 L, three necked flask is placed KBr (10 g, 84 mmol), NaOAc
(10 g, 122 mmol), Compound 1 (50 g, 96 mmol), and TEMPO (15 g, 96 mmol),
followed by 500 mL of MTBE. The reaction mixture is stirred at 350-400
rpm and the temperature is maintained at a temperature of from 10° C. to
20° C. Acetic acid (50 mL, 874 mmol), and water (5 mL) are added to the
reaction mixture and the two phase mixture is agitated for 15 minutes.
Continuously, over a two hour period, to the reaction mixture is added
158 mL of a 0.82 M solution of NaOCl (130 mmol). When all of the NaOCl
solution is added, the reaction mixture is stirred for an additional 3
hours while maintaining the temperature. Water (50 mL) is added.
The layers are separated and the organic layer is washed twice with
water (2×250 mL). A solution of ascorbic acid, which is prepared from 50
g of sodium ascorbate, 200 mL of water, and 50 mL of 4N HCl, is added
to the organic layer and the mixture is stirred for about 1 hour. After
the layers are separated, the organic layer is washed twice with water
(2×250 mL). The organic layer is concentrated by distilling off solvent
at low temperature (0-5° C.) until the total volume is about 350 mL. The
concentrated organic layer is added dropwise over 30 minutes into a 3 L
flask containing 2 L of n-heptane at about 0° C. providing a white
precipitate. The white precipitate is collected by filtration, washed
with n-heptane (400 mL) and dried in a vacuum oven (2 hr at 25° C., 8 hr
at 350, and 8° C. at 45° C.). The product is obtained as a white powder
(typically 94-96% yield).
1H NMR, δ 0.84 (d, J=2.3 Hz, 3H), 0.90-1.02 (m, 9H), 0.99
(d, J=4.0 Hz, 3H), 1.24 (s, 9H), 1.40-1.86 (m, 7H), 1.90-2.10 (m, 3H),
2.25-2.40 (m, 1H), 3.75 (dd, J=5.3 and 10.4 Hz, 1H), 4.10 (dd, J=6.8 and
10.4 Hz, 1H), 4.4 (dd, J=3.0 and 5.3 Hz, 2H), 5.17 (dddd, J=4.6, 8.1,
8.1, and 10.4 Hz, 1H), 5.3 (br s, 2H), 6.71 (d, J=14.7 Hz, 1H), 6.90
(dd, J=2.3 and 19.0 Hz, 1H), and 7.34 (dd, J=7.1 and 20.2 Hz, 1H).
Example 2Preparation of Compound 2 Using Glacial Acetic Acid in the Reaction Mixture
Into a 2 L, three necked flask was charged KBr (20 g, 168 mmol),
NaOAc (20 g, 243 mmol), Compound 1 (100 g, 192 mmol), and TEMPO (30 g,
192 mmol), followed by 800 mL of MTBE. The reaction mixture was stirred
at 350400 rpm while the temperature of the reaction mixture was
maintained at a temperature of from 10° C. to 20° C. Acetic acid (70 mL,
1223 mmol, used as received), was added and the mixture was agitated
for 15 minutes additional. Continuously, over a two hour period, 315 ml
of a 0.73M solution of NaOCl (230 mmol) was added to the reaction
mixture. When all of the NaOCl solution had been added, agitation was
continued for an additional 3 hours. Water (100 mL) was added to the
reaction mixture at the end of 3 hours. The layers were separated and
the organic layer was washed once with water (500 mL).
A solution of ascorbic acid, which was prepared from 100 g of sodium
ascorbate, 456 mL of water, and 44 mL of 36% HCl, was added to the
organic layer and the mixture was stirred for about 2 hours. The layers
were separated and then a solution of 3.5N HCL was added and stirred
about 30 minutes. After the layers were separated, the organic layer was
washed three times with water (3×500 mL). This organic layer was then
added drop-wise over 30 minutes into a 5 L flask containing 3 L of
n-heptane at about −10 to about 0° C. The white precipitate was
filtered, washed with n-heptane (600 mL) and dried in a vacuum oven (2
hr at 25° C., 8 hr at 350, and 8° C. at 45° C.). The product was
obtained as a white powder (93% yield).
1H NMR, δ 0.84 (d, J=2.3 Hz, 3H), 0.90-1.02 (m, 9H), 0.99
(d, J=4.0 Hz, 3H), 1.24 (s, 9H), 1.40-1.86 (m, 7H), 1.90-2.10 (m, 3H),
2.25-2.40 (m, 1H), 3.75 (dd, J=5.3 and 10.4 Hz, 1H), 4.10 (dd, J=6.8 and
10.4 Hz, 1H), 4.4 (dd, J=3.0 and 5.3 Hz, 2H), 5.17 (dddd, J=4.6, 8.1,
8.1, and 10.4 Hz, 1H), 5.3 (br s, 2H), 6.71 (d, J=14.7 Hz, 1H), 6.90
(dd, J=2.3 and 19.0 Hz, 1H), and 7.34 (dd, J=7.1 and 20.2 Hz, 1H).

Boceprevir
…………………


Chinese journal of medicinal chemistry 2011, 21, 5 , pg 409-10


……………………
J Med Chem,2006,49(20):6074-6086.
……………………………………….
WO2004/113294 A1
…………..
MSN LABORATORIES LIMITED; THIRUMALAI RAJAN, Srinivasan; ESWARAIAH,
Sajja; VENKAT REDDY, Ghojala; SAHADEVA REDDY, Maramreddy Patent:
WO2014/61034 A1, 2014 ;
………………………..
WO2013066734A1
MERCK SHARP and DOHME CORP.; WU, George, G.; ITOH, Tetsuji; MCLAUGHLIN, Mark; LIU, Zhijian; QIAN, Gang Patent:
WO2013/66734 A1, 2013 ;
Example 1: Cyclobutylacetonitrile
Step 1 : Cyclobutylmethyl methanesulfonate
A 50-L jacket vessel was charged with DCM (20 L) (KF 34 ppm), and
cyclobutylmethyl alcohol (5.0 kg, 58.0 mol) followed by TEA (8850 mL,
63.5 mol). The reaction mixture was cooled to approximately -10°C, and
MsCl (4735 mL, 60.8 mol) was added via an addition funnel dropwise over
approximately 3 hours, while the temperature was maintained below -5°C.
The reaction resulted in a yellow slurry after 70 minutes of aging. H
20 (8 L) was added to give a clear solution, which was agitated for 15 minutes. Then, the organic layer was separated. H
20
(8 L) was charged to the organic layer. The mixture was agitated for 20
minutes, and then the organic layer was separated. Brine (10% solution,
4 L) was charged to the organic layer. The mixture was agitated for 20
minutes, and then the organic layer was separated. The organic phase was
concentrated by vacuum distillation at approximately 30°C to 40°C and
28 inches Hg, resulting in a light brown residue (10.0 kg crude,
approximately 9.5 kg product assumed, 58.0 mol, approximately 100%
yield). A portion of the material was purified by distillation for
characterization.
1H NMR (CDC1
3, 400 MHz): δ 4.18 (d, J = 6.8 Hz, 2H), 3.00 (s, 3H), 2.71 (m, 1H), 2.11 (m, 2H), 2.00-1.80 (m, 4H).
Step 2: Cyclobutylacetonitrile
A 100-L RB flask was set up with a mechanical stirrer, a thermocouple, an addition funnel, a N
2 inlet,
and a condenser that is connected to a scrubber (11 L bleach and 5 L 2N
NaOH). DMSO (30.3 L) (KF approximately 680 ppm) and NaCN (3030 g, 61.8
mol) were charged to the flask. The mixture was heated to approximately
75 °C by steam to dissolve most chunks of NaCN, resulting in a turbid
solution. The product of Step 1 (9476 g, 57.7 mol) in DMSO (4 L) was
added dropwise in 1 hour, 40 minutes while the temperature was
maintained below approximately 87°C. The reaction was aged at
approximately 85°C for 3 hours and cooled down to RT. H
20 (24
L) and MTBE (24 L) were charged. The mixture was agitated, and the
organic layer was separated. The aqueous layer was extracted with MTBE
(18 L), and the combined organic layer was agitated with H
20
(12 L) and separated. The organic layer was washed with 10% brine (4 L
and 2 L), and concentrated by vacuum distillation at approximately 45°C
and approximately 20 inches Hg, giving a light brown liquid (7.235 kg
crude, 73.3% by GC assay, 5.30 kg product assay, 55.7 mol, 96.5% for two
steps).
Ή NMR (CDCI
3, 400 MHz): δ 2.65 (m, 1H), 2.41 (d, J – 5.2 Hz , 2H), 2.18 (t, J = 6.8 Hz, 2H), 2.00-1.80 (m, 4H).
Example 2: Ethyl 4-cyclobutyl-3-oxobutanoate
THF (20 L) and zinc dust (2.75 kg, 42.0 mol) were charged under N
2 to
a 50-L jacketed vessel with a thermocouple, an addition funnel and a
condenser. The mixture was stirred, and chlorotrimethylsilane (0.571 kg,
5.26 mol) was added at RT. The mixture was heated at 67°C for 30
minutes. Cyclobutylacetonitrile (2.5 kg, 26.3 mol, product of Example 1)
was added at 67°C. Ethyl bromoacetate (6.108 kg, 36.6 mol) was added to
the mixture at approximately 67°C to 70°C for over 3 hours. After the
addition, the mixture was heated at approximately 70°C for 1 hour and
then cooled to approximately 0°C to 5°C. 10% H
2S0
4 (aq.) (35
L, 33.9 mol, approximately 1.3 eq.) was added slowly. The mixture was
aged at RT for 1 hour. The organic layer was separated and subsequently
washed with 10% aqueous citric acid (15 L, 7.88 mol, 0.3 eq.), 10%
aqueous Na
2S
20
5 (25 L), 10% Na
2S
20
5 (aq.)
(10 L), and 10% brine (10 L). The organic layer was concentrated in
vacuo to afford the crude product (4.08 kg assay, 22.15 mol) in 84%
yield. A part of the material was purified by distillation for
characterization (with NMR in CDC1
3, approximately 10-15% enol-form of the compound was observed, major keto-form as shown.)
1H NMR (CDC1
3, 400 MHz): δ 4.19 (q, J = 7.1 Hz, 2 H), 3.38
(s, 2 H), 2.75-2.65 (m, 1H), 2.65-2.63 (m, 2 H), 2.19-2.08 (m, 2 H),
1.95-1.79 (m, 2 H), 1.73-1.60 (m, 2 H), 1.27 (t, J = 7.1 Hz, 3 H).
13C NMR (CDC1
3, 400 MHz): δ 202.2, 167.2, 61.3, 50.0, 49.3, 31.1, 28.4, 18.7,
14.1.
Example 3: Ethyl 2-chloro-4-c clobut l-3-oxobutanoate
Methyl t-butyl ether (30.2 L), and the crude product of Example 2 (3.78 kg assay,
20.52 mol) were charged to a 100-L RB flask with an overhead stirrer,
an addition funnel, a thermometer, and an acid scrubber (with 2N NaOH
at RT under N
2). Sulfuryl chloride (2.98 kg,
22.06 mol) was added at approximately 20°C to 23 °C over 1.5 hours.
After addition, the mixture was cooled to approximately 5°C and then
quenched with 1M K
3P0
4 (aq.) (23.6 L). The organic
layer was separated and concentrated under vacuum to afford the crude
chloride (4.487 kg, assume 100% yield, 20.52 mol), which was used in the
next reaction without purification. A part of the material was purified
by distillation for characterization (with NMR in CDC1
3,
approximately 10% enol-form of the compound was observed, major keto-form was shown below).
1H NMR (CDCI
3, 400 MHz): δ 4.73 (s, 1 H), 4.29 (q, J = 7.1
Hz, 2 H), 2.89-2.79 (m, 2 H), 2.79-2.69 (m, 1 H), 2.20-2.07 (m, 2 H),
1.98-1.78 (m, 2 H), 17.3-1.61 (m, 2 H), 1.32 (t, J = 7.1 Hz, 3 H).
13C NMR (CDC1
3, 400 MHz): δ 198.1, 165.0, 63.1,
60.9, 45.7, 31.0, 28.3, 18.7, 13.9. Example 4: -C clobut l-l-ethox
-l,3-dioxobutan-2-yl 4-methoxybenzoate
The crude chloride product of Example 3 (4.487 kg assumed, 20.52 mol)
and Ν,Ν-dimethylformamide (11.2 L) were charged to a 50-L jacketed
vessel with a thermocouple and a condenser at RT under N
2.
-Methoxybenzoic acid (3.75 kg, 24.62 mol) and TEA (2.285 kg, 22.57 mol)
were added to the mixture. The mixture was heated at 55°C for 14 hours.
The mixture was cooled to approximately 10°C, diluted with methyl
tert-butyl ether (24 L), quenched with ¾0 (24 L). The organic layer was
separated and subsequently washed with IN NaHC0
3 (20 L), then H
20 (18 L) with NaCl (0.90 kg) and NaHC0
3 (0.45
kg). The organic layer was separated and concentrated in vacuo to
afford the product (6.07 kg, 18.15 mol) in 88% assay yield. A part of
the material was purified by distillation for characterization.
1H NMR (CDCI
3, 400 MHz): δ 8.09 (dt, J = 2.1, 9.0 Hz, 2
H), 6.96 (dt, J = 2.1, 9.0 Hz, 2 H), 5.66 (s, 1 H), 4.31 (q, J = 7.1 Hz,
2 H), 3.88 (s, 3 H), 2.86 (dd, J = 5.7, 7.6 Hz, 2 H, 2.83-2.74 (m, 1
H), 2.23-2.12 (m, 2H), 1.98-1.80 (m, 2 H), 1.74-1.65 (m, 2 H), 1.32 (t, J
= 7.1 Hz, 3 H).
Example 5: (2 -3-Amino-4-cyclobutyl-l-ethoxy-l-oxobut-2-en-2-yl 4-methoxybenzoate
The crude product of Example 4 (5.97 kg, 17.85 mol), 1-propanol (12
L), and EtOH (12 L) were charged to a 100-L RB flask with an overhead
stirrer and a thermometer at RT under N
2. NH
4OAc
(4.82 kg, 62.5 mol) was added to the mixture. The mixture was heated at
50°C for 1 hour. The mixture was concentrated in vacuo to remove H
20 azeotropically with continuous addition of 1-propanol (total approximately 24 L). The mixture was solvent-switched to i
‘PrOAc (24 L) under vacuum. The mixture was quenched with 2M K
3P0
4 (aq.)
(17.85 L). The organic layer was separated and washed with 15% brine
(18 L) twice. The organic layer was concentrated in vacuo to afford
crude enamine product (5.95 kg, assume 100% yield, 17.85 mol).
1H NMR (CDC1
3, 400 MHz): δ 8.12 (d, J= 8.0 Hz, 2H), 6.98 (d, J= 8.0 Hz, 2H),
6.02 (s, 2H), 4.15 (q, J= 8 Hz, 2H), 3.89 (s, 3H), 2.60-2.53 (m, 1H), 2.33 (s, 2H), 2.13-2.06 (m,
2H), 1.91-169 (m, 4H), 1.20 (t, J = 8 Hz, 3H).
13C NMR (CDC1
3, 400 MHz): δ 165.7, 167.6, 163.6, 153.9, 132.1, 122.2, 113.9,
113.7, 112.5, 59.6, 44.5, 37.8, 33.9, 28.5, 28.4, 18.5, 14.4.
Example 6A: 3-[(tert-Butoxycarbonyl)amino]-4-cyclobutyl-l-ethoxy-l-oxobut-2-yl 4- methoxybenzoate
The crude product of Example 5 (5.92 kg, 17.75 mol) and MeOH (23.7 L)
were charged to a 100-L RB flask with an overhead stirrer, a
thermocouple, and an addition funnel at RT under N
2. Di-tert-butyl dicarbonate (5.81 kg, 26.6 mol) and sodium cyanoborohydride
(1.171 kg, 18.64 mol) were charged to the mixture. A solution of
glycolic acid (1.485 kg, 19.53 mol) in MeOH (3.55 L) was added to the
mixture drop wise at a rate to maintain the temperature at approximately
15°C to 22°C. The mixture was aged at approximately 20°C for
approximately 8-10 hours. EtOAc (3.49 L, 35.5 mol) and a solution of
glycine (0.866 kg, 11.4 mol) in H
20 (11 L) were added to the mixture at RT. Then, 2M K
3P0
4 (aq ) solution
(17.75 L) was added. The mixture was aged for 20 minutes. The mixture
was extracted with methyl tert-butyl ether (28 L). The organic layer was
separated and washed subsequently with 2M K
3P0
4 (aq.) solution
(17.75 L), 10% brine (17.75 L, twice). The organic layer was
concentrated under vacuum to afford the desired two diastereoisomers in
almost 1 : 1 ratio (7.30 kg, 16.76 mol) in 94% assay yield.
1H NMR (CDCI
3, 400 MHz): δ 8.02 (d, J= 8.0 Hz, 2H), 6.94 (d, J= 8.0 Hz, 1H),
6.93 (d, J= 8.0 Hz, 1H), 5.30 (d, J= 4.0 Hz, 0.5H), 5.17 (d, J= 4.0
Hz, 0.5H), 4.80 (d, J= 8.0 Hz, 0.5H), 4.63 (d, J = 8.0 Hz, 0.5H),
4.27-4.18 (m, 3H), 3.86 (s, 3H), 2.50-2.30 (m, 1H), 2.15- 2.00 (m, 2H),
1.89-1.60 (m, 6H), 1.43 -1.42 (m, 9H), 1.27 (t, J= 8.0 Hz, 3H).
Example 6B: 3-[(tert-Butoxycarbonyl)amino]-4-cyclobutyl-l-ethoxy-l-oxobut-2-yl 4- methoxybenzoate (First alternate procedure)
The crude product of Example 5 (19.2 g, 58.0 mmol) and MeOH (100 mL)
were charged to an autoclave with a thermocouple at RT. Di-tert-butyl
dicarbonate (19.0 g, 87.0 mmol) and 5% Ir/CaC0
3 (10.0 g) were charged to the mixture. The mixture was heated to 40°C under sealed conditions, where H
2 was transferred until the internal pressure became
approximately 200 psig. The mixture was heated at 40°C at
approximately 200 psig for 20 hours. The reaction mixture was cooled to
RT and filtered to remove the solid to afford a clear solution. EtOAc
(5.7 mL, 58 mmol) and a solution of glycine (2.8 g, 38 mmol) in H
20 (37 mL) were added to the mixture at RT. Then, 2M K
3P0
4 (aq ) solution
(58 mL) was added. The mixture was aged for 20 minutes. The mixture was
extracted with methyl tert-butyl ether (130 mL). The organic layer was
separated and washed subsequently with 2M
3P0
4 (aq.) solution
(58 mL), 10% brine (58 mL, twice). The organic layer was concentrated
under vacuum to afford the desired two diastereoisomers in almost 1 :1
ratio (23 g, 52 mmol) in a 90% assay yield.
1H NMR (CDC1
3, 400 MHz): δ 8.02 (d, J= 8.0 Hz, 2H), 6.94
(d, J= 8.0 Hz, 1H), 6.93 (d, J= 8.0 Hz, 1H), 5.30 (d, J= 4.0 Hz, 0.5H),
5.17 (d, J- 4.0 Hz, 0.5H), 4.80 (d, J= 8.0 Hz, 0.5H), 4.63 (d, J= 8.0
Hz, 0.5H), 4.27-4.18 (m, 3H), 3.86 (s, 3H), 2.50-2.30 (m, 1H), 2.15-
2.00 (m, 2H), 1.89-1.60 (m, 6H), 1.43 -1.42 (m, 9H), 1.27 (t, J= 8.0 Hz,
3H). Example 6C:
3-[(tert-Butoxycarbonyl)amino]-4-cyclobutyl-l-ethoxy-l-oxobut-2-yl 4-
methoxybenzoate (Second alternate procedure)
NaBH
4 (0.23 g, 6 mmol) and THF (5 mL) were charged to a
100-ml RB flask. The mixture was cooled to -10°C. Methanesulfonic acid
(0.78 mL, 12 mmol) was charged slowly into the mixture at less than -8°C
and the mixture was agitated for 15 minutes. A 0.3M solution of the
crude product of Example 5 (1 g, 3 mmol) in THF was charged slowly into
the mixture at below -8°C. The mixture was agitated for 16 hours. H
20
(1 ml) was charged slowly into the mixture at 0°C, and the mixture was
warmed to RT. Di-tert-butyl dicarbonate (1.31 g, 6 mmol) and 2M aqueous
NaOH (3.75 ml) were charged into the mixture. The mixture was agitated
for 2 hours at RT. An assay of the reaction mixture gave the product
(1.23 g, 94%). Example 7A: Ethyl
3-f(tert-buyoxycarbonyl)aminoJ-4-cyclobutyl-2-hydroxybutanoate
The crude product of Example 6A (6.0 kg, 13.78 mol) and MeOH (24 L)
were charged into a 10-gallon autoclave at RT. The mixture was heated to
70°C under sealed conditions, where NH
4 was transferred
until the internal pressure became approximately 80 psig. The mixture
was heated at 70°C at approximately 80 psig for 22 hours. The mixture
was cooled to RT. NH
4 was vented at RT. DMSO (5.4 L) was
added to the mixture, and the mixture was aged at RT for 1 hour. The
mixture was transferred into a 100-L RB flask with an overhead stirrer
and a thermometer. The autoclave was rinsed with MeOH, and the mixture
and rinse liquid were combined. This combined mixture was concentrated
to remove MeOH under vacuum. Then, the flask was rinsed with DMSO (2.6
L) to wash the walls. Total DMSO volume was 8.0 L. The mixture was
heated to 70°C to dissolve the solid to afford a clear solution, which
was cooled to RT slowly to afford a slurry. ¾0 (32.0 L) was charged for
approximately 1.5 hours at 20°C to 27°C. After addition of H
20,
the mixture was aged at RT overnight and then cooled to 0°C to 5°C for 4
more hours. The mixture was filtered to collect the solid, which was
washed with cold H
20 (12 L). The solid was dried at 40°C in a vacuum oven with N
2 sweep (approximately 150 torr) to afford the crude product 5.63 kg (3.75 kg).
1H NMR (DMSO-d
6, 400 MHz): δ 7.20-7.15 (m, 2H), 7.25 (d,
J= 12.0 Hz, 0.5H), 5.92 (d, J= 12.0 Hz, 0.44H), 5.52-5.44 (m, 1H),
3.83-3.81 (m, 0.5H), 3.74-3.62 (m, 1.5H), 2.29- 2.22 (m, 1H), 2.03-1.92
(m, 2H), 1.83-1.70 (m, 2H), 1.62-1.24 (m, 13H).
13C NMR (DMSO-d
6, 400 MHz) δ 175.2, 174.6,
155.5, 155.4, 78.0, 77.9, 74.4, 72.7, 51.9, 51.8, 38.8, 35.8, 33.3,
33.2, 33.0, 28.8, 28.7, 28.6, 28.5, 28.4, 28.2, 18.6, 18.5.
Example 7B: Ethyl 3-[(tert-buyoxycarbonyl)amino]-4-cyclobutyl-2-hydroxybutanoate
The crude product of Example 6A (6.0 g, 84 wt%, 11.57 mmol) and CaCl
2 (1.413 g, 12.73 mmol) and 7N NH
3 in
MeOH (60 mL, 420 mmol) were charged into a 40 mL vial. The mixture was
aged at approximately 33°C for 3 hours. The mixture was concentrated
under reduced pressure to afford the product (7.8 g crude, assume 100%
yield) as a tan solid. Example 8: Ethyl
3-amino-4-cyclobutyl-2-hydroxybutanoate hydrochloride
IP A (13.8 L) was charged into a 100-L RB flask with a mechanical
stirrer, dry and clean with a thermometer and an addition funnel,
followed by addition of the product of Example 7 (3.46 kg assay, 12.70
mol). HCI in IPA (5-6 M 13.8 L, 69 mol) was slowly added into the
reaction mixture. The reaction mixture was heated at 50°C for 4 hours.
The mixture was cooled to RT. Then, MTBE (28 L) was added to the mixture
over 30 minutes. The reaction mixture was cooled to 0°C to 5°C by
MeOH/ice bath for 1.5 hour. The mixture was filtered to collect the
solid, which was washed with MTBE (7 L) twice. The wet cake was dried
under vacuum with N
2 and sweep overnight to afford the product as an off-white solid (2.15 kg, 10.30 mol) in 76.6% overall yield for Examples 5-8.
1H NMR (DMSO-d
6, 400 MHz): δ 8.20-7.95 (m, 3H), 7.54-7.44
(m, 2H), 6.46 (d, J= 4.0 Hz, 0.5H), 6.26 (d, J= 8.0 Hz, 0.5H), 4.22 (s,
0.5H), 3.98 (s, 0.5H), 3.26 (s, 0.5H), 3.10 (d, J= 4.0 Hz, 0.5H),
2.45-2.36 (m, 1H), 2.00-1.96 (m, 2H), 1.81-1.39 (m, 6H).
1
3C NMR (DMSO-d
6, 400 MHz) δ 174.1, 173.6, 71.2, 69.8, 51.7, 51.5, 36.0, 34.6,
31.7, 31.5, 28.0, 27.8, 27.7, 18.3, 18.1.
Exam le 9: Ethyl 3-amino-4-cyclobutyl-2-hydroxybutanoate hydrochloride (Recrystallization)
H
20 (3.0 L), CH
3CN (6 L) and the product of
Example 8 (2.00 kg, 9.58 mol) were charged to a 100-L RB flask with an
overhead stirrer, a thermocouple and a condenser at RT under N
2. The mixture was heated to 65°C to get a clear solution. The mixture was cooled to 50°C to get a thin slurry. CH
3CN (6.0 L) was added at 50°C for over 1 hour. The mixture was cooled to 40°C. CH
3CN (9.0 L) was added at 40°C for over 1 hour. The mixture was cooled to 30°C. CH
3CN
(18 L) was added at 30°C. The mixture was cooled to approximately 0°C
to 5°C and stirred for 1 hour before filtration. The mixture was
filtered, washed with CH
3CN (4 L) twice, and dried with N
2 stream to afford the recrystallized product as a white solid (1.887 kg, 9.04 mol, 94% isolated yield).
Ή NMR (DMSO-d
6, 400 MHz): δ 8.20-7.95 (m, 3H), 7.54-7.44
(m, 2H), 6.46 (d, J= 4.0 Hz, 0.5H), 6.26 (d, J= 8.0 Hz, 0.5H), 4.22 (s,
0.5H), 3.98 (s, 0.5H), 3.26 (s, 0.5H), 3.10 (d, J= 4.0 Hz, 0.5H),
2.45-2.36 (m, 1H), 2.00-1.96 (m, 2H), 1.81-1.39 (m, 6H).
13C NMR (DMSO-d
6, 400 MHz): δ 174.1, 173.6, 71.2, 69.8, 51.7, 51.5, 36.0, 34.6, 31.7, 31.5, 28.0, 27.8, 27.7, 18.3, 18.1.
Example 10:
(lR,2S,5S)-N-(4-amino-l-cyclobutyl-3-hydroxy-4-oxobutan-2-yl)-3-[N-(t
rt- butylcarbamoyl)-3-methyl-I^valyl]-6,6-dimethyl-3-azabicyclo[3A ]h
Hydroxybenzotiazole (HOBT, 4.83 g, 31.5 mmol), water (4.5 mL),
(1R,2S,5S)-N- (4-amino- 1 -cyclobutyl-3 -hydroxy-4-oxobutan-2-yl)-3-
[N-(tertbutylcarbamoyl)-3 -methylvalyl] –
6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide (30 g, 60.6 mmol),
HCl salt product of Example 9 (13.79 g, 66.1 mmol), ethyl acetate (120
mL) and N-methyl-2-pyrrolidone (NMP, 30 mL) were added at 19°C to a
three-necked 500mL RB flask equipped with an overhead stirrer and a
thermocouple. N-methylmorpholine (13.3 mL, 121 mmol) was added to the
mixture at 19°C. l-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDCI,
15.0 g, 78.0 mmol) was added to the mixture at 21°C. Ethyl acetate (30
mL) was then added to the mixture at 18°C.
The mixture was agitated at approximately 20°C to 24°C for about 16
hours. After the reaction was complete, ethyl acetate (120 mL) was added
at 23°C. The mixture was washed with 10% aqueous potassium carbonate
solution (180 mL) twice at approximately 20°C to 24°C. Then, the organic
layer was washed with 3.3% aqueous HCl (180 mL) twice at approximately
12°C to 18°C. The organic layer then was washed with 10% aqueous
potassium carbonate solution (180 mL) and water (180 mL). The organic
layer was concentrated to approximately 100 mL volume and was added to
heptane (900 mL) dropwise at approximately -10°C to -5°C to precipitate
the product. The mixture was filtered and washed with heptane. The solid
was dried in vacuo at approximately 50°C to 60°C overnight. 31.3 g of
the product compound was obtained as a white solid in 99% yield. The
above procedure is in accordance with the processes disclosed in U.S.
Patent Application Publication No. US2010/519485 Al, the disclosures of
which are herein
incorporated by reference. It will be appreciated that the processes
disclosed therein can be modified without undue experimentation to
prepare specifically desired materials. The results of H NMR and C NMR
for the above procedure were consistent with those reported in U.S.
Patent Application Publication No. US2010/519485 Al .
Example 11:
(lR,5S)-N-[3-Amino-l-(cyclobutylmethyl)-2,3-dioxopropyl]-3-[2(S)-[[[(l,l-
dimethylethyl)amino]carbonyl]amino]-3,3-dimethyl-l-oxobutyl]-6,6-dimazabicyclo[3.1.0]hexan-2(S)-carboxamide
Acetic acid (27.0 mL, 472 mmol) and MTBE (240 mL) at RT were added to
a three-necked 1L RB flask equipped with an overhead stirrer, a
thermocouple and a chiller. The mixture was cooled to approximately
14°C, then the product from Example 10 (30.0 g, 57.5 mmol) was charged
at approximately 14°C. The mixture was cooled to approximately 11°C.
2,2,6,6-Tetramethylpiperidin-l-yl)oxyl (TEMPO, 9.97 g, 63.8 mmol) was
added to the mixture. A pre-mixed solution containing 40% aqueous sodium
permanganate (17.02 g, 48.0 mmol) and water (99 mL) at approximately
12°C to 14°C was added to the reaction mixture over about 2 hours. The
mixture was agitated at approximately 12°C until completion.
After the reaction was complete, the mixture was cooled to
approximately 1°C. Water (30 mL) was added, then aqueous layer was
separated. The organic layer was then washed with water (150 mL) at
approximately 0°C to 10°C, and then washed with a pre-mixed solution of
sodium ascorbate (30.0 g, 151 mmol) in water (150 mL) and concentrated
HCl (12.42 mL, 151 mmol) at approximately 5°C to 15°C. The mixture was
agitated at approximately 5°C to 10°C for 2 hours; then aqueous layer
was separated. The organic layer was further washed with 2.5 N HCl (120
mL) at approximately 0°C to 10°C and with water (150 mL) at
approximately 0°C to 10°C four times. The organic layer
(approximately 170 mL) was then added dropwise to heptane (720 mL) at
approximately -20°C to -15°C to precipitate the product. The mixture was
then warmed to -5°C and filtered to collect the solid. The solid was
washed with heptane, dried in a vacuum oven with nitrogen sweep at room
temperature to afford 27.1 g of desired product of Formula II as a white
solid in 91% yield.
The above procedure is in accordance with the processes disclosed in U.S.
Provisional Patent Application No.61/482,592 (unpublished), the
disclosures of which are herein incorporated by reference. It will be
appreciated that the processes disclosed therein can be modified without
undue experimentation to prepare specifically desired materials. The
results of 1H NMR and
13C NMR for the above procedure were
consistent with those reported in U.S. Provisional Patent Application
No.61/482,592 (unpublished).
………………………………………………………………
see part 2 at.........
http://orgspectroscopyint.blogspot.in/2015/08/boceprevir-part-22.html
see more at
http://www.allfordrugs.com/2015/08/02/boceprevir-%D0%B1%D0%BE%D1%86%D0%B5%D0%BF%D1%80%D0%B5%D0%B2%D0%B8%D1%80-%D8%A8%D9%88%D8%B3%D9%8A%D8%A8%D8%B1%D9%8A%D9%81%D9%8A%D8%B1-%E6%B3%A2%E6%99%AE%E7%91%9E%E9%9F%A6/
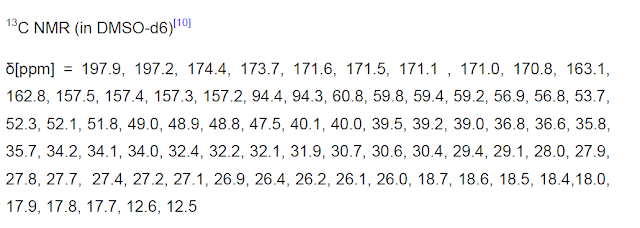
![[14C]-Boceprevir NMR spectra analysis, Chemical CAS NO. 394730-60-0 NMR spectral analysis, [14C]-Boceprevir H-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2014-11-29/002/493/2493725_1h.png)


![[14C]-Boceprevir NMR spectra analysis, Chemical CAS NO. 394730-60-0 NMR spectral analysis, [14C]-Boceprevir H-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2014-11-29/002/493/2493725_1h.png)
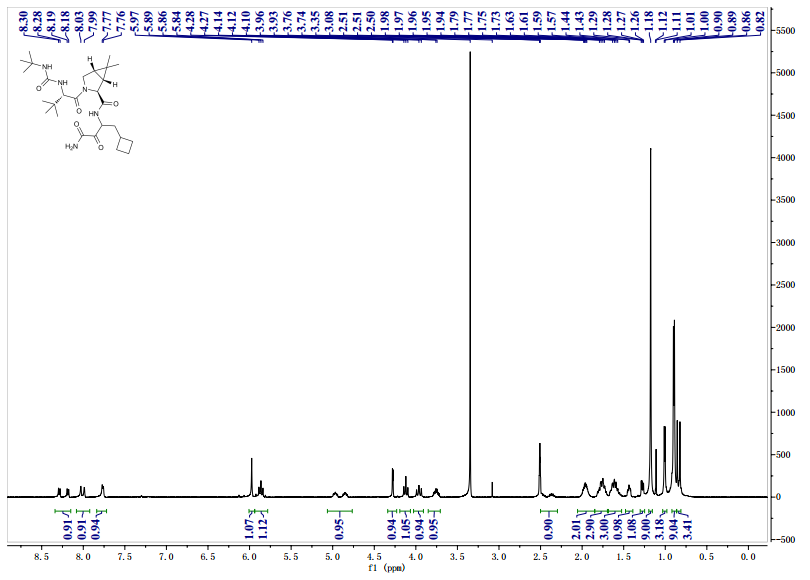
![[14C]-Boceprevir NMR spectra analysis, Chemical CAS NO. 394730-60-0 NMR spectral analysis, [14C]-Boceprevir C-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2014-11-29/002/493/2493725_13c.png)