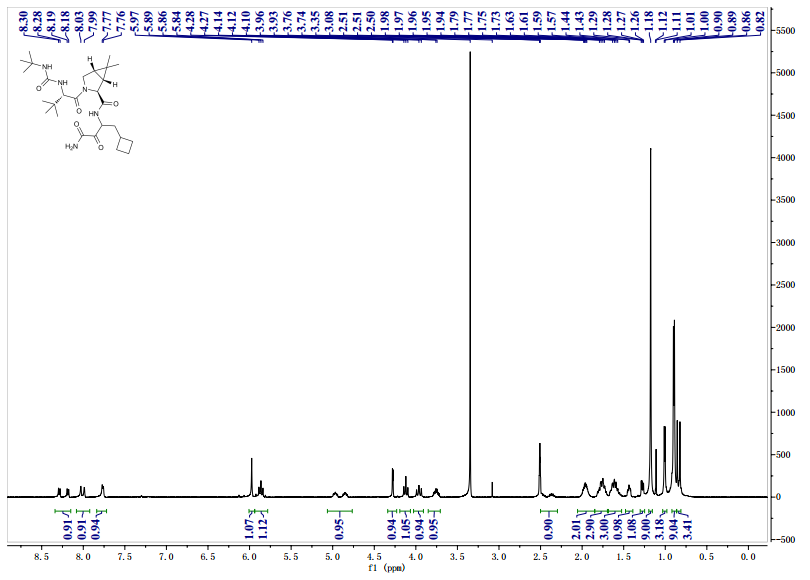
![[14C]-Boceprevir NMR spectra analysis, Chemical CAS NO. 394730-60-0 NMR spectral analysis, [14C]-Boceprevir H-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2014-11-29/002/493/2493725_1h.png)
……………………………………………………………….
EXTRAS,,,,,,,,,,see http://www.allfordrugs.com/2015/08/02/boceprevir-%D0%B1%D0%BE%D1%86%D0%B5%D0%BF%D1%80%D0%B5%D0%B2%D0%B8%D1%80-%D8%A8%D9%88%D8%B3%D9%8A%D8%A8%D8%B1%D9%8A%D9%81%D9%8A%D8%B1-%E6%B3%A2%E6%99%AE%E7%91%9E%E9%9F%A6/
1H NMR GRAPH


13c nmr
13 C NMR GRAPH
WILL BE UPDATED
![[14C]-Boceprevir NMR spectra analysis, Chemical CAS NO. 394730-60-0 NMR spectral analysis, [14C]-Boceprevir H-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2014-11-29/002/493/2493725_1h.png)
13C NMR PREDICT
![[14C]-Boceprevir NMR spectra analysis, Chemical CAS NO. 394730-60-0 NMR spectral analysis, [14C]-Boceprevir C-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2014-11-29/002/493/2493725_13c.png)
| WO2010138889A1* | 28 May 2010 | 2 Dec 2010 | Concert Pharmaceuticals, Inc. | Peptides for the treatment of hcv infections |
| WO2011125006A2* | 31 Mar 2011 | 13 Oct 2011 | Pfizer Inc. | Novel sultam compounds |
| US20110034705 * | 17 Dec 2008 | 10 Feb 2011 | Schering-Plough Corporation | Process For the Synthesis of 3- Amino-3-Cyclobuthylmethyl-2-Hydroxypropionamide or Salts Thereof |
| US8188137 | 14 Aug 2009 | 29 May 2012 | Avila Therapeutics, Inc. | HCV protease inhibitors and uses thereof |
| US8524760 | 10 Apr 2012 | 3 Sep 2013 | Celgene Avilomics Research, Inc. | HCV protease inhibitors and uses thereof |
| EP2704570A1 * | 2 May 2012 | 12 Mar 2014 | Merck Sharp & Dohme Corp. | Drug substances, pharmeceutical compositions and methods for preparing the same |
| WO2014061034A1* | 17 Oct 2013 | 24 Apr 2014 | Msn Laboratories Limited | Process for preparation of boceprevir and intermediates thereof |
References
- 1 Kiser JJ, Burton JR, Anderson PL, Everson GT (May 2012). “Review and Management of Drug Interactions with Boceprevir and Telaprevir”. Hepatology 55 (5): 1620–8. doi:10.1002/hep.25653. PMC 3345276. PMID 22331658.
- 2
- Degertekin B, Lok AS (May 2008). “Update on viral hepatitis: 2007″. Curr. Opin. Gastroenterol. 24 (3): 306–11.doi:10.1097/MOG.0b013e3282f70285. PMID 18408458.
- 3
- Njoroge FG, Chen KX, Shih NY, Piwinski JJ (January 2008). “Challenges in modern drug discovery: a case study of boceprevir, an HCV protease inhibitor for the treatment of hepatitis C virus infection”. Acc. Chem. Res. 41 (1): 50–9. doi:10.1021/ar700109k.PMID 18193821.
- 4
- “Boceprevir – FDA Antiviral Drugs”. FDA. FDA. April 2011. Retrieved April 2014.
- 5
- “Interim Results from Boceprevir Phase II Study in Genotype 1 Treatment-Naive Hepatitis C Patients Presented At EASL – Forbes.com” (Press release). Forbes.com. Retrieved 2008-05-19.
- 6
- “FDA Approves Merck’s VICTRELIS™ (boceprevir), First-in-Class Oral Hepatitis C Virus (HCV) Protease Inhibitor” (Press release). Merck & Co. Retrieved 2011-05-14.
- 7
- http://www.hcvadvocate.org/news/reports/EASL_2009/Advocate_EASL_2009_Coverage.htm
- 8
- Poordad, F et al. (March 2011). “Boceprevir for Untreated Chronic HCV Genotype 1 Infection”. N Engl J Med. 364 (13): 1195–206. doi:10.1056/NEJMoa1010494. PMID 21449783.
- 9
- Jensen, D (March 2011). “A New Era of Hepatitis C Therapy Begins”. N Engl J Med. 364 (13): 1272–1273.doi:10.1056/NEJMe1100829. PMID 21449791.
- 10
| SYSTEMATIC (IUPAC) NAME | |
|---|---|
| (1R,5S)-N-[3-Amino-1-(cyclobutylmethyl)-2,3-dioxopropyl]-3-[2(S)-[[[(1,1-dimethylethyl)amino]carbonyl]amino]-3,3-dimethyl-1-oxobutyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2(S)-carboxamide | |
| CLINICAL DATA | |
| TRADE NAMES | Victrelis |
| AHFS/DRUGS.COM | Consumer Drug Information |
| MEDLINEPLUS | a611039 |
| LICENCE DATA | US FDA:link |
| |
| |
| Oral | |
| PHARMACOKINETIC DATA | |
| PROTEIN BINDING | 75% [1] |
| HALF-LIFE | 3.4 hours [1] |
| IDENTIFIERS | |
| 394730-60-0 | |
| J05AE12 | |
| PUBCHEM | CID 10324367 |
| CHEMSPIDER | 8499830 |
| UNII | 89BT58KELH |
| CHEMBL | CHEMBL218394 |
| NIAID CHEMDB | 398493 |
| CHEMICAL DATA | |
| FORMULA | C27H45N5O5 |
////

No comments:
Post a Comment