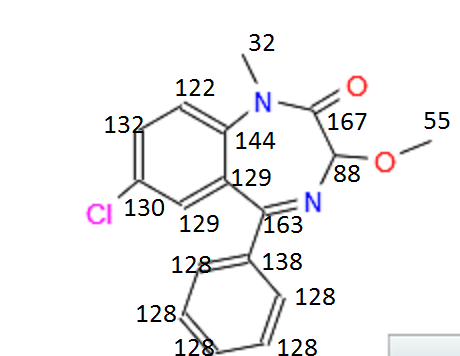
[(3RS)-7-chloro-3-methoxy-1-methyl-5-phenyl-1,3-dihydro-2h-1,4-benzodiazepin-2-one]
Temazepam (20 g; 0.067 mol) and methanol
(120 ml) was added to a 0.5 Lt, 4 necked flask bearing a mechanical
stirrer, addition flask and thermometer, set up on a cooling tub at room
temperature. Perchloric acid
(70%; 2mL (10%/vol.) was added slowly through a dropping funnel keeping
the reaction temperature between 20-25°C in 1-2 h. The reaction mixture
was allowed to come to room temperature and was stirred overnight. Then
distilled complete methanol under reduced pressure and residue adding to 50% Na2CO3 solution (200 ml). The reaction mass was extracted with CH2Cl2 (2 X 200 ml) and dried over Na2SO4. The dichloromethane
was concentrated under reduced pressure to obtain a light yellow oily
liquid which on forced scratching with glass rod gives light yellow
solid (18 g, 86%).
IR (cm-1): 1680.00 ([C=O]), 1608.00 ([HCOCH3]) (ether).
1H NMR (CDCl3; 400MHz):
δ 7.68-7.66(d;2H;ArH),7.56-7.47(m;2H;ArH),7.44-7.40(t;2H;ArH),7.35-7.32(t;2H;ArH), 4.67(s;1H,COCH),3.63(s;3H;NCH3),3.43(s;3H;OCH3).
13C NMR (CDCl3; 100 MHz):167.40, 163.92, 141.57, 137.17, 131.88, 130.97, 130.20, 129.63, 129.53, 129.42, 128.38, 122.89, 90.02, 55.38, 35.19.
1H NMR (CDCl3; 400MHz):
δ 7.68-7.66(d;2H;ArH),7.56-7.47(m;2H;ArH),7.44-7.40(t;2H;ArH),7.35-7.32(t;2H;ArH), 4.67(s;1H,COCH),3.63(s;3H;NCH3),3.43(s;3H;OCH3).
13C NMR (CDCl3; 100 MHz):167.40, 163.92, 141.57, 137.17, 131.88, 130.97, 130.20, 129.63, 129.53, 129.42, 128.38, 122.89, 90.02, 55.38, 35.19.
IR graph
1H NMR GRAPH
/////////




No comments:
Post a Comment