The NMR Spectra of Alkenes seem to have some very strange properties that defy explanation by the simple N+1 rule. In this article we will learn:
1. The Difference in NMR Spectra for cis and trans Alkenes.
2. What Coupling Constants (J Values) are.
3. Violations of the Simple N+1 Rule.
4. How to interpret the NMR Spectra of Alkenes.
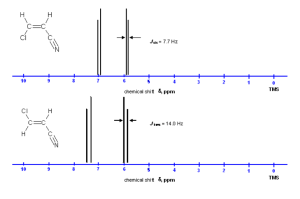
NMR Spectra of Cis vs. Trans Alkenes
Based on what we’ve discussed about NMR spectra so far you would think that the cis and trans isomers of alkenes would have identical spectra.
In fact, based on the example above this turns out not to be true. The distance between the peaks, called the coupling constant J, is not the same for cis and trans alkenes.
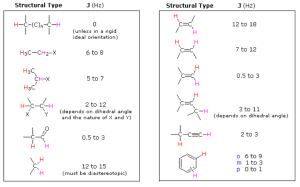
Coupling Constants (J Values)
Coupling constants (J values) are defined as the interpeak distance in a particular peak in a NMR spectra. They depend on the distance and angle between the coupled protons.
If you really want to be able to master this stuff it helps to know ballpark numbers for each of these J values in the table above.
Violations of the Simple N+1 Rule
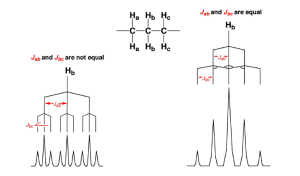
Let’s say a proton is linked to 4 other protons. By the simple N+1 rule we would expect to see its spectra split into a quintet. However, it turns out that the simple N+1 rule only holds if the J values for each of the linked protons is the same.
Let’s see what happens if the J values aren’t the same:
The example on the right is what we’d expect to see if the J ab and J bc values were the same. However, because they are not we must apply the compound N+1 rule.
The compound N+1 rule says that when we have different coupling constants we apply the N+1 rule sequentially to each. So, for example our protons labeled as Hb are split into triplets by the Ha protons and then split again into triplets by the Hc protons, creating a triplet of triplets.
Guess where else we see the compound N+1 rule? That’s right, in cis / trans systems for alkenes.
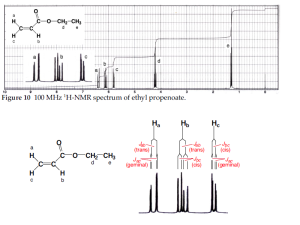
NMR Spectra of Alkenes
Let’s analyze protons a, b and c.
Ha: Split by Hb (J geminal = 0.5-3 Hz from above) and by Hc (J trans = 12 -18 Hz from above). Because they’re different we apply the compound N+1 rule and get a doublet of doublets.
Hb: Split by Ha (J trans = 12-18 Hz) and Hc (J cis = 7-12 Hz). Of the three It looks most like a doublet of doublets since it has the largest J values (peaks are more spaced out)
Hc: Split by Ha (J geminal = 0.5-3 Hz) and Hb (J cis = 7-12 Hz). Also adoublet of doublets, and very similar to Ha except the peaks are closer since J cis < J trans.
This type of system is very common amongst alkenes, read more about it here.
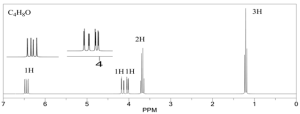
Example 1
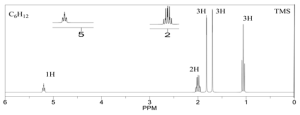
1. Calculate the DoU and Hypothesize:
DoU = 1
Most probably an alkene because of the signal at 5 ppm.
2. Write the pieces:
1.0 ppm (t, 3H) CH3
1.6 ppm (s, 3H) CH3
1.7 ppm (s, 3H) CH3
2.0 ppm (multiplet, 2H) CH2
5.3 ppm (triplet, 1H) CH
3. Structural units:
CH3-CH2-
CH3-
CH3-
CH=C
4. Subtract from Molecular Formula:
Everything’s accounted for.
5. Assemble:
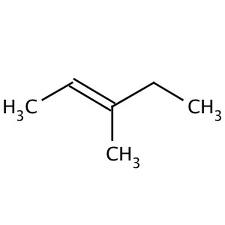
Two possible ways to arrange the pieces from above -
Which one is it? The multiplet at 2 ppm tells us the answer. In the structure on the left the CH2 group should be split into a doublet of quartets. In the structure on the right it would be split into a doublet. It must be the structure on the left.
6. Verify:
The chemical shifts and splitting patterns look correct!
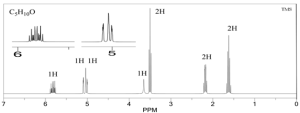
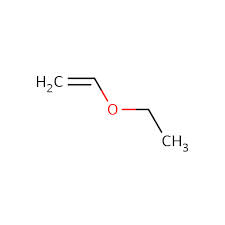
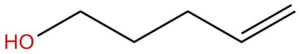
Example 2
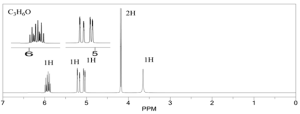
1. Calculate DoU and Hypothesize:
DoU = 1
Probably an alcohol because the singlet at 3.5 ppm is most likely the OH proton.
2. Write the pieces:
3.5 ppm (s, 1H) OH
4.1 ppm (d, 2H) CH2
5.1 ppm (dd, 1H) CH
5.3 ppm (dd, 1H) CH
5.9 (multiplet, 1H) CH
3. Structural Units:
The last 3 peaks are all part of a double bond: CH=CH2
CH2
OH
4. Subtract from Molecular Formula:
We’ve accounted for everything.
5. Assemble:
6. Verify:
Looks good, the 2 Hydrogens on the end of the alkene would be a doublet of doublets and the internal hydrogen is a doublet of doublet of triplets.
Examples for Further Practice
Example 3 -
Example 4 -
Conclusion
We’ve learned four things today:
1. The Difference in NMR Spectra for cis and trans Alkenes – Trans alkenes have larger interpeak distances because of greater J values.
2. What Coupling Constants (J Values) are – J values are the interpeak distance for a set of coupled protons and depend on the distance and angle between those protons in the molecule.
3. Violations of the Simple N+1 Rule – If a proton is coupled to 2 or more sets of protons with different J values, you have to apply the compound N+1 rule and treat each set of protons separately.
4. How to interpret the NMR Spectra of Alkenes – Alkenes generally have different J values, so we see the compound N+1 rule usually applied, creating patterns like doublets of doublets.
















DACCA, BANGLADESH
Farmer Harvesting Jute, Tangail,Dhaka, Bangladesh Photographic Print
////////////