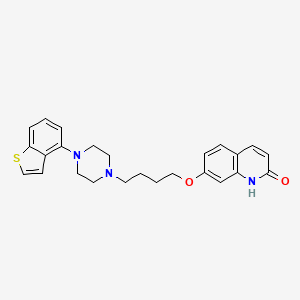
Brexpiprazole
ブレクスピプラゾール
OPC-34712, UNII-2J3YBM1K8C, OPC34712,
CAS 913611-97-9,
Molecular Weight:433.56578 g/mol
7-[4-[4-(1-benzothiophen-4-yl)piperazin-1-yl]butoxy]-1H-quinolin-2-one
7-[4-[4-(1-Benzothiophen-4-yl)piperazin-1-yl]butoxy]quinolin-2(1H)-one
2(1H)-Quinolinone, 7-[4-(4-benzo[b]thien-4-yl-1-piperazinyl)butoxy]-
7- [ 4- ( 4-benzo[b]thiophen-4- yl-piperazin-l-yl)butoxy] -lH-quinolin-2-one
7-[4-(4-benzo[b]thiophen-4-yl-piperazin-1-yl)butoxy]-1H-quinolin-2-one
NDA is considered filed as of September 9, 2014 (60 days after submission). The PDUFA date is July 11, 2015.
 Brexpiprazole
Brexpiprazole (
// breks-pip-rə-zohl; also called
OPC-34712) is a novel
D2 dopamine partial
agonist investigational product currently in clinical trials for the treatment of
depression,
schizophrenia, and
attention deficit hyperactivity disorder(ADHD).
[1]Although it failed Stage 2 trials for ADHD, it has been designed to provide improved efficacy and tolerability (e.g., less
akathisia,
restlessness and/or
insomnia) over established adjunctive treatments for
major depressive disorder (MDD).
[2]
OPC-34712 is an antidepressant and antipsychotic drug candidate
awaiting approval in the U.S. for the treatment of schizophrenia and
also as adjunctive treatment of major depressive disorder (MDD). The
product is in phase III clinical trials for the treatment of agitation
associated with Alzheimer’s disease. Phase III clinical trials are also
underway for the treatment of post-traumatic stress disorder (PTSD).
brexpiprazole (pre-registration, as of April 2015), which is being
developed by Otsuka and Lundbeck, useful for treating schizophrenia,
agitation associated with Alzheimer’s disease, major depressive disorder
and attention deficit hyperactivity disorder. Family members of the
product case, WO2006112464, hold protection in EU states until 2026 and
its US equivalent, US7888362, has US154 extension, expiring in 2027.
Suzhou Vigonvita Life Sciences appears to be new to patenting and is the
first collaborative filing from the three assignees.

Phase II clinical trials are also ongoing for use as adjunctive
therapy in adults with attention deficit hyperactivity disorder (ADHD).
The compound is being developed by Otsuka Pharmaceutical. In 2011, a
codevelopment and commercialization agreement was signed by Lundbeck and
Otsuka Pharmaceutical in Latin and North America, Australia and Europe
for the treatment of psychiatric disorders.
The drug is being developed by
Otsuka, and is considered to be a successor
[3] of its top-selling antipsychotic agent
aripiprazole(brand names:
Abilify,
Aripiprex). Otsuka’s US patent on aripiprazole expired on October 20, 2014;
[4] however, due to a pediatric extension, a generic will not become available until at least April 20, 2015.
[5]

Brexpiprazole (
1) , a serotonin–dopamine activity modulator,
is an investigational new drug currently in phase-III clinical trials
for the treatment of depression, schizophrenia, and attention deficit
hyperactivity disorder.
(1A) Brexpiprazole is also considered to be a possible successor to the top-selling antipsychotic agent aripiprazole.
(2A)
-
-
Brexpiprazole
In the clinical program, brexpiprazole demonstrated improvement in
symptoms in both schizophrenia and as adjunctive therapy in major
depressive disorder (MDD)
July 2015 is the anticipated completion timing of the FDA’s review (based on PDUFA timeline)Otsuka
Pharmaceutical Co., Ltd. (Otsuka) and H. Lundbeck A/S (Lundbeck) today
announced that the U.S. Food and Drug Administration (FDA) has
determined that the New Drug Application (NDA) for brexpiprazole for
monotherapy in adult patients with schizophrenia and for adjunctive
treatment of major depressive disorder (MDD) in adult patients is
sufficiently complete to allow for a substantive review, and the NDA is
considered filed as of September 9, 2014 (60 days after submission). The
PDUFA date is July 11, 2015.The NDA is supported by seven completed
placebo-controlled clinical phase II or III studies in the proposed
indications – three studies in schizophrenia and four studies with
brexpiprazole as adjunctive therapy in MDD. The dossier included data
from more than 6,000 participants of whom more than 5,000 received
brexpiprazole.
Brexpiprazole in adult patients with schizophreniaOne
clinical phase II and two clinical phase III placebo-controlled studies
have been completed using brexpiprazole in adult patients suffering
from schizophrenia. Across the three studies more than 1,700 patients
have been randomized.In the first pivotal phase III study randomizing
approximately 625 patients, brexpiprazole 2mg/day and 4 mg/day both
demonstrated greater improvement of symptoms relative to placebo as
measured by change from baseline in the Positive and Negative Syndrome
Scale (PANSS) Total Score at week 6 (p<0.05). Results of the key
secondary endpoint supported primary results.In the second pivotal phase
III study randomizing approximately 650 patients, brexpiprazole 4
mg/day again demonstrated greater improvement of symptoms relative to
placebo (p<0.05) in change from baseline in the PANSS Total Score at
Week 6. Brexpiprazole 2 mg/day showed numerical improvement (p>0.05)
over placebo at Week 6.The results from the clinical phase II studyi
were presented at the 24th Annual US Psychiatric and Mental Health
Congress in November 2011. The study showed a clinically meaningful
improvement from baseline measured by PANSS total score at week 6,
although it did not achieve statistical separation from placeboii.In the
placebo-controlled phase II and III studies, the rates of
discontinuation due to adverse events were 8.1% for patients receiving
brexpiprazole compared to 12.7% of patients receiving placebo; the only
adverse event that occurred in more than 5% of brexpiprazole patients
and more frequently than placebo was akathisia (5.8% vs. 4.5%).
Brexpiprazole as adjunctive therapy in major depressive disorder (MDD) Four
studies have been included in the dossier using brexpiprazole as
adjunctive therapy for adult patients suffering from MDD who had
demonstrated a consistent, inadequate response to at least two regimens
of prior antidepressant treatment. Patients with MDD and an inadequate
response to one to three antidepressants were enrolled and received
antidepressants for 8 weeks, single blinded, in the two phase III
studies. Patients with an inadequate response during this prospective
phase were provided antidepressant therapy and randomized adjunctive
treatment with either brexpiprazole or placebo for 6 weeks. The primary
efficacy endpoint was the change in MADRS (Montgomery–Åsberg Depression
Rating Scale) Total Score from baseline at week 6. MADRS is a commonly
used scale to assess the range of symptoms in patients with MDD. Across
the four studies, more than 3,900 patients entered the prospective phase
and more than 1,800 patients were included in the randomized phase of
the studies.The first pivotal phase III results were presented in a
poster session at the 22nd European Psychiatry Association Congress
(EPA) in March 2014. This two-arm phase III study randomized
approximately 380 patients and demonstrated an improvement of symptoms
with an antidepressant plus 2 mg brexpiprazole that was greater than an
antidepressant plus placebo (p<0.001)The second pivotal phase III
study was a three-arm study in which approximately 675 patients were
randomized to treatment with an antidepressant plus either placebo, 1 mg
brexpiprazole or 3 mg brexpiprazole.v Patients in both brexpiprazole
treatment groups showed greater improvement in symptoms as measured by
the MADRS compared to placebo (1 mg p>0.05, 3 mg p<0.05). Results
of the second pivotal phase III study in MDD have not yet been
published.
The first clinical phase IIvi study randomized
approximately 425 patients in four arms and was presented at the 164th
Annual Meeting of the American Psychiatric Association in May 2011.
Patients exhibited greater improvements than adjunctive placebo in MADRS
Total score with the 1.5 (±0.5) mg/day dose of brexpiprazole after six
weeks of treatment (p
About brexpiprazole (OPC-34712)Brexpiprazole
is a novel investigational psychotropic compound discovered by Otsuka
and under co-development with Lundbeck. Brexpiprazole is a
serotonin-dopamine activity modulator (SDAM) that acts as a partial
agonist at 5-HT1A and dopamine D2 receptors at similar potency, and an
antagonist at 5-HT2A and noradrenaline alpha1B/2C receptors.

Partnership with Lundbeck
In November 2011,
Otsuka and
Lundbeck have announced a global alliance.
[6]
Lundbeck has given Otsuka an upfront payment of $200 million, and the
deal includes development, regulatory and sales payments, for a
potential total of $1.8 billion. Specifically for OPC-34712, Lundbeck
will obtain 50% of net sales in Europe and Canada and 45% of net sales
in the US from Otsuka.
The partnership has been presented by Otsuka to its investors as a good fit for several reasons:
[7]
- Geographic strategy: Otsuka in Japan, Asia, US; Lundbeck in Europe, South America and emerging markets
- Research strategy: Otsuka has knowledge in antipsychotics, Lundbeck in anti-depressant and anxiolytic.
- CNS strategy: Otsuka has a robust portfolio in next-generation CNS
drugs, while Lundbeck covers a wide range of CNS conditions from
Alzheimer’s to schizophrenia.
- Similar corporate culture

Clinical trials
OPC-34712 is currently in clinical trials for adjunctive treatment of MDD, adjunctive treatment of adult ADHD and schizophrenia.
[8]
Major depression
Phase II
The Phase 2 multicenter, double-blind, placebo-controlled study
randomized 429 adult MDD patients who exhibited an inadequate response
to one to three ADTs in the current episode. The study was designed to
assess the efficacy and safety of OPC-34712 as an adjunctive treatment
to standard ADT. The ADTs included in the study were desvenlafaxine,
escitalopram, fluoxetine, paroxetine, sertraline, and venlafaxine.
[9]
Phase III
A new Phase III study is currently in the recruiting stage: “Study of
the Safety and Efficacy of Two Fixed Doses of OPC-34712 as Adjunctive
Therapy in the Treatment of Adults With Major Depressive Disorder (the
Polaris Trial)”.
[10]
Its goal is “to compare the effect of OPC-34712 to the effect of
placebo (an inactive substance) as add on treatment to an assigned FDA
approved antidepressant treatment (ADT) in patients with Major
Depressive Disorder who demonstrate an incomplete response to a
prospective trial of the same assigned FDA approved ADT”. Estimated
enrollment is 1250 volunteers.
Adult ADHD
Phase II
- Study of the Safety and Efficacy of OPC-34712 as a Complementary
Therapy in the Treatment of Adult Attention Deficit/Hyperactivity
Disorder (STEP-A)[11] The company did not move the product to Phase III, and it is presumed this drug failed Phase II trials for the disorder.
Schizophrenia
Phase I
- Trial to Evaluate the Effects of OPC-34712 on QT/QTc in Subjects With Schizophrenia or Schizoaffective Disorder[12]
Phase II
- A Dose-finding Trial of OPC-34712 in Patients With Schizophrenia[13]
Phase III
- Efficacy Study of OPC-34712 in Adults With Acute Schizophrenia (BEACON)[14]
- Safety and Tolerability Study of Oral OPC-34712 as Maintenance Treatment in Adults With Schizophrenia (ZENITH)[15]
- Study of the Effectiveness of Three Different Doses of OPC-34712 in the Treatment of Adults With Acute Schizophrenia (VECTOR)[16]
- A Long-term Trial of OPC-34712 in Patients With Schizophrenia[17]
Conferences
- Phase II results were presented at the American Psychiatric Association’s 2011 annual meeting in May 2011.[18]
- The drug has been presented at the 2nd Congress of Asian College of Neuropsychopharmacology[19] in September 2011.
- At the US Psychiatric and Mental Health Congress in November 2011 in
Vegas, Robert McQuade presented the Phase II Trial results for
Schizophrenia[20]
Side effects
The most common adverse events associated with OPC-34712 (all doses
of OPC-34712 cumulatively greater than or equal to 5 percent vs.
placebo) were upper respiratory tract infection (6.9% vs. 4.8%),
akathisia (6.6% vs. 3.2%), weight gain (6.3% vs. 0.8%), and
nasopharyngitis (5.0% vs. 1.6%).
[21]
Drug interactions
Based on information given on the consent forms, it seems OPC-34712 is a substrate of
CYP2D6 and
CYP3A4,
like its predecessor Aripiprazole. Participants in the clinical trials
are advised to avoid grapefruit, Seville oranges and related citruses.
Pharmacology
Brexpiprazole acts as a
partial agonist of the
5-HT1A,
D2, and
D3 receptors, and as an
antagonist of the
5-HT2A,
5-HT2B,
5-HT7,
α1A–,
α1B–,
α1D–, and
α2C-adrenergic, and
H1receptors.
[22] It has negligible
affinity for the
mACh receptors.
[22]
Dosage
- As an adjunct to standard antidepressant therapy in adult patients with major depressive disorder:
- Phase II trials: 1.5 ± 0.5 mg.
- Phase III trials: 1 or 3 mg depending on group.[10]
- For schizophrenic/schizoaffective subjects, dosage is 4 or 12 mg.[23]
- For ADHD, the dose was thought to be 0.25 to 2 mg/day.[11]
Patents
- U.S. Patent 8,071,600
- WIPO PCT/JP2006/317704
- Canadian patent: 2620688[24]
- WO 2013162046
- WO 2013161830
- WO 2013162048
- WO 2013015456
- JP 2008115172
- WO 2006112464
| Patent |
Submitted |
Granted |
| PIPERAZINE-SUBSTITUTED BENZOTHIOPHENES FOR TREATMENT OF MENTAL DISORDERS [US2011152286] |
2011-06-23 |
|
| Piperazine-substituted benzothiophenes for treatment of mental disorders [US7888362] |
2010-07-15 |
2011-02-15 |
Synthesis
WO 2013015456
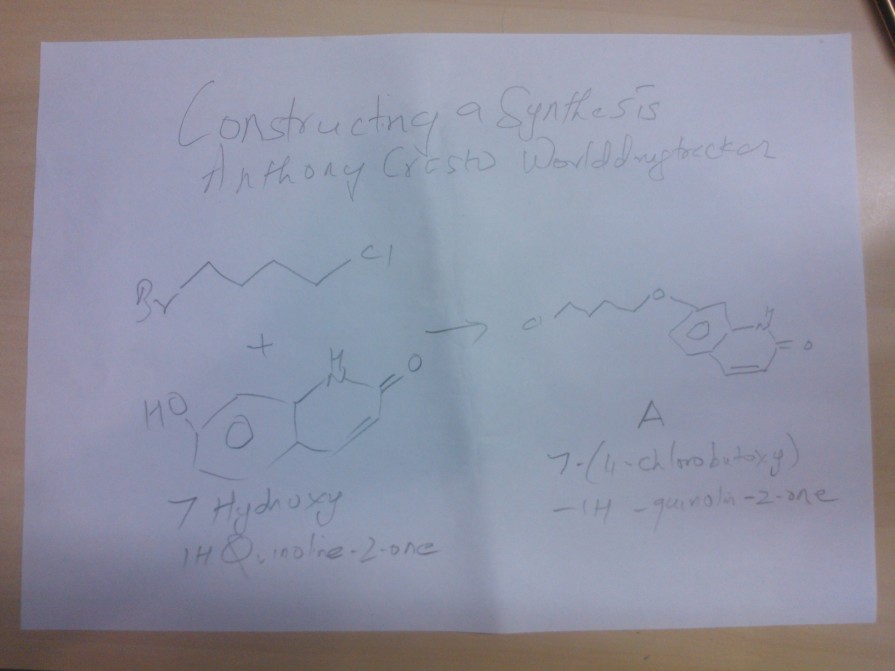
IN THIS BELOW PIC WE SEE
click on pics below to view
Synthesis of A
1 BROMO 4 CHLORO BUTANE WAS REACTED WITH 7 HYDROXY 1H QUINOLINE -2-ONE TO GIVE A
7 ( 4 CHLORO BUTOXY)-1H -QUINOLINE-2-ONE, WHICH WILL BE USED FOR COUPLING AT LAST STAGE

1 BROMO 4 CHLORO BUTANE
IN THE BELOW PIC 2,6-Dichlorobenzaldehyde AND RHODANINE WERE REACTED TO GIVE 2,6-dichlorobenzylidenerhodanine.

2,6-Dichlorobenzaldehyde

RHODANINE
NEXT WAS
2,6-dichlorobenzylidenerhodanine, GAVE (Z)-3-(2,6-dichlorophenyl)-2-mercapto-2-propenoic acid.
1H-NMR (DMSO-d6) d
ppm; 7.23-7.67 (4H, m), 3.5-5.7 (1H, br.), 11.7-14.5 (1H, br.).
Next was prepration of K salt
(Z)-3-(2,6-dichlorophenyl-2-mercapto-2-propenoic acid and potassium
hydroxide gave ((Z)-3-(2,6-dichlorophenyl-2-mercapto-2-propenoic acid
potassium salt).
Next stage
((Z)-3-(2,6-dichlorophenyl-2-mercapto-2-propenoic acid potassium
salt) GAVE 2-carboxy-4-chlorobenzo[b]thiophene.
Yield: 48.8 g. 1H-NMR (DMSO-d6) d ppm; 7.53 (1H, t, J = 7.7 Hz), 7.58 (1H, dd, J = 7.7, 1.3
Hz), 8.03 (1H, d, J = 0.5 Hz), 8.07 (1H, d, J = 7.6 Hz).
NEXT IS DECARBOXYLATION
A mixture of 2-carboxy-4-chlorobenzo[b]thiophene, 1,3-dimethyl-2-imidazolidinone, and 1,8-
diazabicyclo[5.4.0]-undec-7-ene GAVE compound. 4-chlorobenzo[b]thiophene. 1H-NMR (DMSO-d6) d ppm; 7.38 (1H, t, J = 8.4
Hz), 7.51 (1H, dd, J = 5.5, 0.8 Hz), 7.48 (1H, dd, J = 7.7, 0.9 Hz), 7.94 (1H, dd, J = 5.5, 0.4
Hz), 8.02 (1H, dt, J = 8.0, 0.9 Hz).
Suzhou, Jiangsu


 .
.