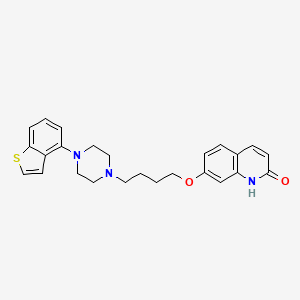
Brexpiprazole
ブレクスピプラゾール
OPC-34712, UNII-2J3YBM1K8C, OPC34712,
CAS 913611-97-9,
Molecular Formula:C25H27N3O2S
Molecular Weight:433.56578 g/mol
7-[4-[4-(1-benzothiophen-4-yl)piperazin-1-yl]butoxy]-1H-quinolin-2-one
7-[4-[4-(1-Benzothiophen-4-yl)piperazin-1-yl]butoxy]quinolin-2(1H)-one
2(1H)-Quinolinone, 7-[4-(4-benzo[b]thien-4-yl-1-piperazinyl)butoxy]-
7- [ 4- ( 4-benzo[b]thiophen-4- yl-piperazin-l-yl)butoxy] -lH-quinolin-2-one
7-[4-(4-benzo[b]thiophen-4-yl-piperazin-1-yl)butoxy]-1H-quinolin-2-one
| Otsuka Pharma Co Ltd, |
Brexpiprazole (/brɛksˈpɪprəzoʊl/ breks-pip-rə-zohl; also called OPC-34712) is a novel D2 dopamine partial agonist investigational product currently in clinical trials for the treatment of depression, schizophrenia, and attention deficit hyperactivity disorder(ADHD).[1]Although it failed Stage 2 trials for ADHD, it has been designed to provide improved efficacy and tolerability (e.g., lessakathisia, restlessness and/or insomnia) over established adjunctive treatments for major depressive disorder (MDD).[2]
OPC-34712 is an antidepressant and antipsychotic drug candidate awaiting approval in the U.S. for the treatment of schizophrenia and also as adjunctive treatment of major depressive disorder (MDD). The product is in phase III clinical trials for the treatment of agitation associated with Alzheimer’s disease. Phase III clinical trials are also underway for the treatment of post-traumatic stress disorder (PTSD).
brexpiprazole (pre-registration, as of April 2015), which is being developed by Otsuka and Lundbeck, useful for treating schizophrenia, agitation associated with Alzheimer’s disease, major depressive disorder and attention deficit hyperactivity disorder. Family members of the product case, WO2006112464, hold protection in EU states until 2026 and its US equivalent, US7888362, has US154 extension, expiring in 2027. Suzhou Vigonvita Life Sciences appears to be new to patenting and is the first collaborative filing from the three assignees.
Phase II clinical trials are also ongoing for use as adjunctive therapy in adults with attention deficit hyperactivity disorder (ADHD). The compound is being developed by Otsuka Pharmaceutical. In 2011, a codevelopment and commercialization agreement was signed by Lundbeck and Otsuka Pharmaceutical in Latin and North America, Australia and Europe for the treatment of psychiatric disorders.
The drug is being developed by Otsuka, and is considered to be a successor[3] of its top-selling antipsychotic agent aripiprazole(brand names: Abilify, Aripiprex). Otsuka’s US patent on aripiprazole expired on October 20, 2014;[4] however, due to a pediatric extension, a generic will not become available until at least April 20, 2015.[5]
Brexpiprazole (1) , a serotonin–dopamine activity modulator, is an investigational new drug currently in phase-III clinical trials for the treatment of depression, schizophrenia, and attention deficit hyperactivity disorder.(1A) Brexpiprazole is also considered to be a possible successor to the top-selling antipsychotic agent aripiprazole.(2A)
-
1A……….Phase II and Phase III Drugs in U.S. Development for Depression, Anxiety, Sleep Disorders, Psychosis & ADHD, 2011. http://www.neurotransmitter.net/newdrugs.html(accessed Jan 27, 2015).
-
2A…………FDA accepts new schizophrenia drug filing, 2014.http://www.pharmafile.com/news/194878/fda-accepts-new-schizophrenia-drug-filing(accessed Jan 27, 2015).BrexpiprazoleIn the clinical program, brexpiprazole demonstrated improvement in symptoms in both schizophrenia and as adjunctive therapy in major depressive disorder (MDD)
July 2015 is the anticipated completion timing of the FDA’s review (based on PDUFA timeline)Otsuka Pharmaceutical Co., Ltd. (Otsuka) and H. Lundbeck A/S (Lundbeck) today announced that the U.S. Food and Drug Administration (FDA) has determined that the New Drug Application (NDA) for brexpiprazole for monotherapy in adult patients with schizophrenia and for adjunctive treatment of major depressive disorder (MDD) in adult patients is sufficiently complete to allow for a substantive review, and the NDA is considered filed as of September 9, 2014 (60 days after submission). The PDUFA date is July 11, 2015.The NDA is supported by seven completed placebo-controlled clinical phase II or III studies in the proposed indications – three studies in schizophrenia and four studies with brexpiprazole as adjunctive therapy in MDD. The dossier included data from more than 6,000 participants of whom more than 5,000 received brexpiprazole.Brexpiprazole in adult patients with schizophreniaOne clinical phase II and two clinical phase III placebo-controlled studies have been completed using brexpiprazole in adult patients suffering from schizophrenia. Across the three studies more than 1,700 patients have been randomized.In the first pivotal phase III study randomizing approximately 625 patients, brexpiprazole 2mg/day and 4 mg/day both demonstrated greater improvement of symptoms relative to placebo as measured by change from baseline in the Positive and Negative Syndrome Scale (PANSS) Total Score at week 6 (p<0.05). Results of the key secondary endpoint supported primary results.In the second pivotal phase III study randomizing approximately 650 patients, brexpiprazole 4 mg/day again demonstrated greater improvement of symptoms relative to placebo (p<0.05) in change from baseline in the PANSS Total Score at Week 6. Brexpiprazole 2 mg/day showed numerical improvement (p>0.05) over placebo at Week 6.The results from the clinical phase II studyi were presented at the 24th Annual US Psychiatric and Mental Health Congress in November 2011. The study showed a clinically meaningful improvement from baseline measured by PANSS total score at week 6, although it did not achieve statistical separation from placeboii.In the placebo-controlled phase II and III studies, the rates of discontinuation due to adverse events were 8.1% for patients receiving brexpiprazole compared to 12.7% of patients receiving placebo; the only adverse event that occurred in more than 5% of brexpiprazole patients and more frequently than placebo was akathisia (5.8% vs. 4.5%).Brexpiprazole as adjunctive therapy in major depressive disorder (MDD) Four studies have been included in the dossier using brexpiprazole as adjunctive therapy for adult patients suffering from MDD who had demonstrated a consistent, inadequate response to at least two regimens of prior antidepressant treatment. Patients with MDD and an inadequate response to one to three antidepressants were enrolled and received antidepressants for 8 weeks, single blinded, in the two phase III studies. Patients with an inadequate response during this prospective phase were provided antidepressant therapy and randomized adjunctive treatment with either brexpiprazole or placebo for 6 weeks. The primary efficacy endpoint was the change in MADRS (Montgomery–Åsberg Depression Rating Scale) Total Score from baseline at week 6. MADRS is a commonly used scale to assess the range of symptoms in patients with MDD. Across the four studies, more than 3,900 patients entered the prospective phase and more than 1,800 patients were included in the randomized phase of the studies.The first pivotal phase III results were presented in a poster session at the 22nd European Psychiatry Association Congress (EPA) in March 2014. This two-arm phase III study randomized approximately 380 patients and demonstrated an improvement of symptoms with an antidepressant plus 2 mg brexpiprazole that was greater than an antidepressant plus placebo (p<0.001)The second pivotal phase III study was a three-arm study in which approximately 675 patients were randomized to treatment with an antidepressant plus either placebo, 1 mg brexpiprazole or 3 mg brexpiprazole.v Patients in both brexpiprazole treatment groups showed greater improvement in symptoms as measured by the MADRS compared to placebo (1 mg p>0.05, 3 mg p<0.05). Results of the second pivotal phase III study in MDD have not yet been published.The first clinical phase IIvi study randomized approximately 425 patients in four arms and was presented at the 164th Annual Meeting of the American Psychiatric Association in May 2011. Patients exhibited greater improvements than adjunctive placebo in MADRS Total score with the 1.5 (±0.5) mg/day dose of brexpiprazole after six weeks of treatment (p
About brexpiprazole (OPC-34712)Brexpiprazole is a novel investigational psychotropic compound discovered by Otsuka and under co-development with Lundbeck. Brexpiprazole is a serotonin-dopamine activity modulator (SDAM) that acts as a partial agonist at 5-HT1A and dopamine D2 receptors at similar potency, and an antagonist at 5-HT2A and noradrenaline alpha1B/2C receptors.
Partnership with Lundbeck
In November 2011, Otsuka and Lundbeck have announced a global alliance.[6] Lundbeck has given Otsuka an upfront payment of $200 million, and the deal includes development, regulatory and sales payments, for a potential total of $1.8 billion. Specifically for OPC-34712, Lundbeck will obtain 50% of net sales in Europe and Canada and 45% of net sales in the US from Otsuka.The partnership has been presented by Otsuka to its investors as a good fit for several reasons:[7]
- Geographic strategy: Otsuka in Japan, Asia, US; Lundbeck in Europe, South America and emerging markets
- Research strategy: Otsuka has knowledge in antipsychotics, Lundbeck in anti-depressant and anxiolytic.
- CNS strategy: Otsuka has a robust portfolio in next-generation CNS drugs, while Lundbeck covers a wide range of CNS conditions from Alzheimer’s to schizophrenia.
- Similar corporate culture
Clinical trials
OPC-34712 is currently in clinical trials for adjunctive treatment of MDD, adjunctive treatment of adult ADHD and schizophrenia.[8]Major depression
Phase II
The Phase 2 multicenter, double-blind, placebo-controlled study randomized 429 adult MDD patients who exhibited an inadequate response to one to three ADTs in the current episode. The study was designed to assess the efficacy and safety of OPC-34712 as an adjunctive treatment to standard ADT. The ADTs included in the study were desvenlafaxine, escitalopram, fluoxetine, paroxetine, sertraline, and venlafaxine.[9]Phase III
A new Phase III study is currently in the recruiting stage: “Study of the Safety and Efficacy of Two Fixed Doses of OPC-34712 as Adjunctive Therapy in the Treatment of Adults With Major Depressive Disorder (the Polaris Trial)”.[10] Its goal is “to compare the effect of OPC-34712 to the effect of placebo (an inactive substance) as add on treatment to an assigned FDA approved antidepressant treatment (ADT) in patients with Major Depressive Disorder who demonstrate an incomplete response to a prospective trial of the same assigned FDA approved ADT”. Estimated enrollment is 1250 volunteers.Adult ADHD
Phase II
- Study of the Safety and Efficacy of OPC-34712 as a Complementary Therapy in the Treatment of Adult Attention Deficit/Hyperactivity Disorder (STEP-A)[11] The company did not move the product to Phase III, and it is presumed this drug failed Phase II trials for the disorder.
Schizophrenia
Phase I
- Trial to Evaluate the Effects of OPC-34712 on QT/QTc in Subjects With Schizophrenia or Schizoaffective Disorder[12]
Phase II
- A Dose-finding Trial of OPC-34712 in Patients With Schizophrenia[13]
Phase III
- Efficacy Study of OPC-34712 in Adults With Acute Schizophrenia (BEACON)[14]
- Safety and Tolerability Study of Oral OPC-34712 as Maintenance Treatment in Adults With Schizophrenia (ZENITH)[15]
- Study of the Effectiveness of Three Different Doses of OPC-34712 in the Treatment of Adults With Acute Schizophrenia (VECTOR)[16]
- A Long-term Trial of OPC-34712 in Patients With Schizophrenia[17]
Conferences
- Phase II results were presented at the American Psychiatric Association’s 2011 annual meeting in May 2011.[18]
- The drug has been presented at the 2nd Congress of Asian College of Neuropsychopharmacology[19] in September 2011.
- At the US Psychiatric and Mental Health Congress in November 2011 in Vegas, Robert McQuade presented the Phase II Trial results for Schizophrenia[20]
Side effects
The most common adverse events associated with OPC-34712 (all doses of OPC-34712 cumulatively greater than or equal to 5 percent vs. placebo) were upper respiratory tract infection (6.9% vs. 4.8%), akathisia (6.6% vs. 3.2%), weight gain (6.3% vs. 0.8%), and nasopharyngitis (5.0% vs. 1.6%).[21]Drug interactions
Based on information given on the consent forms, it seems OPC-34712 is a substrate of CYP2D6 and CYP3A4, like its predecessor Aripiprazole. Participants in the clinical trials are advised to avoid grapefruit, Seville oranges and related citruses.Pharmacology
Brexpiprazole acts as a partial agonist of the 5-HT1A, D2, and D3 receptors, and as an antagonist of the 5-HT2A, 5-HT2B, 5-HT7, α1A–, α1B–, α1D–, and α2C-adrenergic, and H1receptors.[22] It has negligible affinity for the mACh receptors.[22]Dosage
- As an adjunct to standard antidepressant therapy in adult patients with major depressive disorder:
- Phase II trials: 1.5 ± 0.5 mg.
- Phase III trials: 1 or 3 mg depending on group.[10]
- For schizophrenic/schizoaffective subjects, dosage is 4 or 12 mg.[23]
- For ADHD, the dose was thought to be 0.25 to 2 mg/day.[11]
Patents
- U.S. Patent 8,071,600
- WIPO PCT/JP2006/317704
- Canadian patent: 2620688[24]
- WO 2013162046
- WO 2013161830
- WO 2013162048
- WO 2013015456
- JP 2008115172
- WO 2006112464
| Patent | Submitted | Granted |
|---|---|---|
| PIPERAZINE-SUBSTITUTED BENZOTHIOPHENES FOR TREATMENT OF MENTAL DISORDERS [US2011152286] | 2011-06-23 | |
| Piperazine-substituted benzothiophenes for treatment of mental disorders [US7888362] | 2010-07-15 | 2011-02-15 |
Synthesis
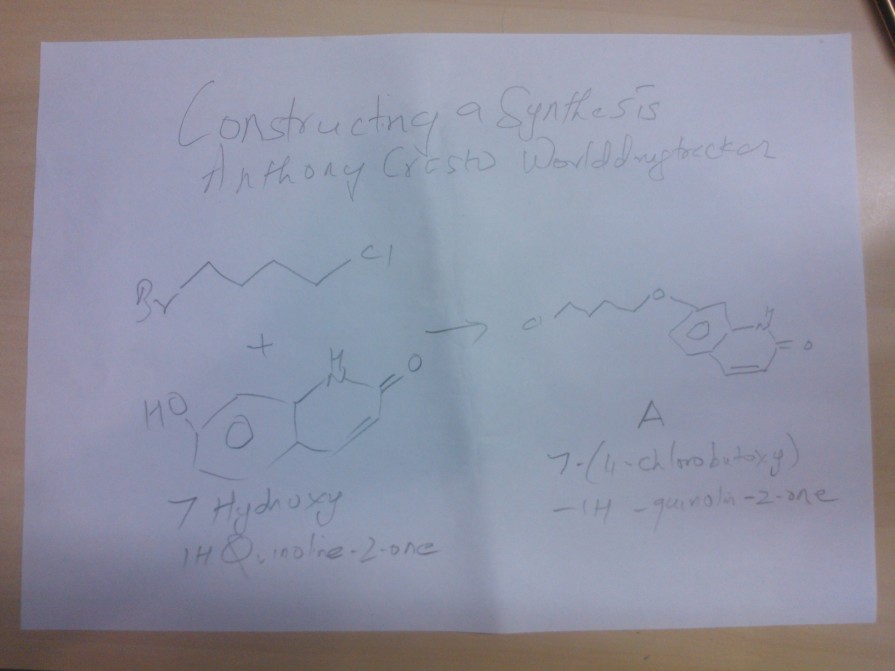
WO 2013015456IN THIS BELOW PIC WE SEE
click on pics below to view
Synthesis of A
1 BROMO 4 CHLORO BUTANE WAS REACTED WITH 7 HYDROXY 1H QUINOLINE -2-ONE TO GIVE A
7 ( 4 CHLORO BUTOXY)-1H -QUINOLINE-2-ONE, WHICH WILL BE USED FOR COUPLING AT LAST STAGE
IN THE BELOW PIC 2,6-Dichlorobenzaldehyde AND RHODANINE WERE REACTED TO GIVE 2,6-dichlorobenzylidenerhodanine.
NEXT WAS
2,6-dichlorobenzylidenerhodanine, GAVE (Z)-3-(2,6-dichlorophenyl)-2-mercapto-2-propenoic acid.
1H-NMR (DMSO-d6) d
ppm; 7.23-7.67 (4H, m), 3.5-5.7 (1H, br.), 11.7-14.5 (1H, br.).
Next was prepration of K salt
(Z)-3-(2,6-dichlorophenyl-2-mercapto-2-propenoic acid and potassium hydroxide gave ((Z)-3-(2,6-dichlorophenyl-2-mercapto-2-propenoic acid potassium salt).
Next stage
((Z)-3-(2,6-dichlorophenyl-2-mercapto-2-propenoic acid potassium
salt) GAVE 2-carboxy-4-chlorobenzo[b]thiophene.
Yield: 48.8 g. 1H-NMR (DMSO-d6) d ppm; 7.53 (1H, t, J = 7.7 Hz), 7.58 (1H, dd, J = 7.7, 1.3
Hz), 8.03 (1H, d, J = 0.5 Hz), 8.07 (1H, d, J = 7.6 Hz).
NEXT IS DECARBOXYLATION
A mixture of 2-carboxy-4-chlorobenzo[b]thiophene, 1,3-dimethyl-2-imidazolidinone, and 1,8-
diazabicyclo[5.4.0]-undec-7-ene GAVE compound. 4-chlorobenzo[b]thiophene. 1H-NMR (DMSO-d6) d ppm; 7.38 (1H, t, J = 8.4
Hz), 7.51 (1H, dd, J = 5.5, 0.8 Hz), 7.48 (1H, dd, J = 7.7, 0.9 Hz), 7.94 (1H, dd, J = 5.5, 0.4
Hz), 8.02 (1H, dt, J = 8.0, 0.9 Hz).