Green Chem., 2017, Advance Article
DOI: 10.1039/C7GC02211E, Paper
DOI: 10.1039/C7GC02211E, Paper
F. A. Kucherov, K. I. Galkin, E. G. Gordeev, V. P. Ananikov
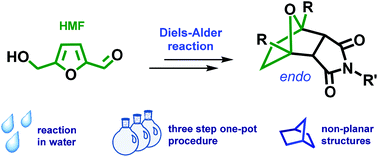
Efficient one-pot synthesis of tricyclic compounds from biobased 5-hydroxymethylfurfural (HMF) is described using a [4 + 2] cycloaddition reaction.
Efficient one-pot synthesis of tricyclic compounds from biobased 5-hydroxymethylfurfural (HMF) is described using a [4 + 2] cycloaddition reaction.
Efficient route for the construction of polycyclic systems from bioderived HMF
Abstract
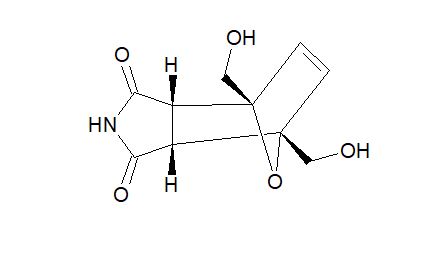
The first synthesis of tricyclic compounds from biobased 5-hydroxymethylfurfural (HMF) is described. The Diels–Alder reaction was used to implement the transition from HMF to a non-planar framework, which possessed structural cores of naturally occurring biologically active compounds and building blocks of advanced materials. A one-pot, three-step sustainable synthesis in water was developed starting directly from HMF. The reduction of HMF led to 2,5-bis(hydroxymethyl)furan (BHMF), which could be readily involved in the Diels–Alder cycloaddition reaction with HMF-derived maleimide, followed by hydrogenation of the double bond. The described transformation was diastereoselective and proceeded with a good overall yield. The applicability of the chosen approach for the synthesis of analogous structures containing amine functionality on the side chain was demonstrated. To produce the target compounds, only platform chemicals were used with carbohydrate biomass as the single carbon source.
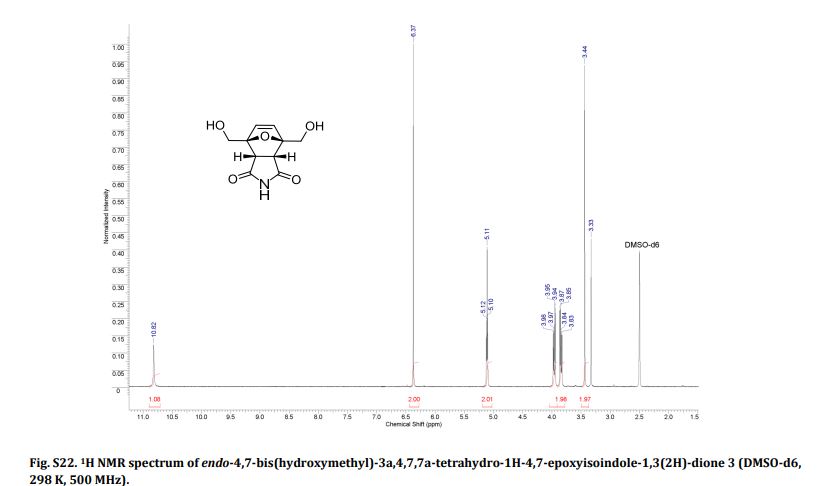
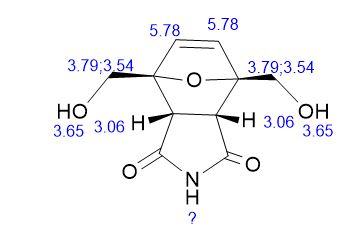
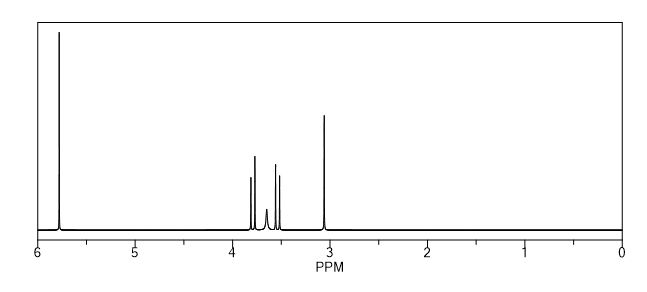
Endo-4,7-bis(hydroxymethyl)-3a,4,7,7a-tetrahydro-1H-4,7-epoxyisoindole-1,3(2H)-dione (endo-4,7-bis(hydroxymethyl)norcantharimid-5-ene), 31H NMR (DMSO-d6) = 10.82 (s, 1H), 6.37 (s, 2H), 5.11 (t, 2H, J = 5.7 Hz), 3.97 (dd, 2H, J = 5.7 Hz, 12.8 Hz), 3.84 (dd, 2H, J = 5.7 Hz, 12.8 Hz), 3.44 (s, 2H);
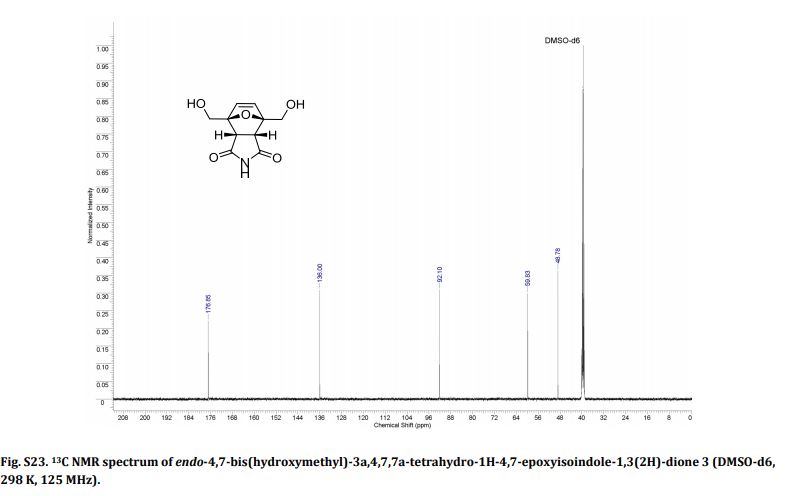
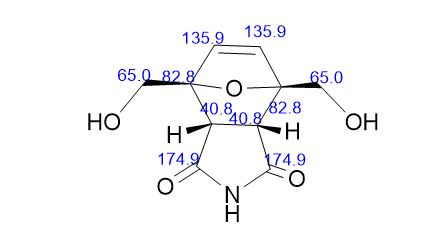
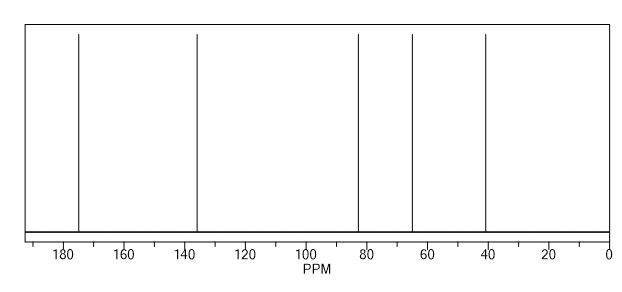
13C NMR (DMSO-d6) = 176.9, 136.0, 92.1, 59.8, 48.8 ppm.
m/z HRMS (ESI) Calcd. for C10H11NO5 [M+Na]: 248.0529. Found 248.0536.